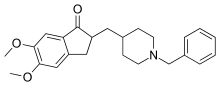
Donepezil
 | |
| Names | |
|---|---|
| Trade names | Aricept, others |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) Absorption is not affected by food or time of day.[1] |
| Onset of action | Peak plasma levels in 3–4 h.[2][1] |
| Duration of action | With daily dosing, steady-state concentration is reached in 15–21 days.[2][1] |
| Defined daily dose | 7.5 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697032 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100%[1][2] |
| Protein binding | 96%, albumin (about 75%) and alpha1-acid glycoprotein (21%).[2][1] |
| Metabolism | CYP2D6, CYP3A4, and glucuronidation.[1] Four major metabolites, two of which are active.[2][1] |
| Elimination half-life | 70 hours[4] Around 100 hours in elderly patients.[1] |
| Excretion | 0.11–0.13 (L/h/kg); excreted mostly by the kidneys. Around 17% is excreted unchanged in the urine. About 15% to 20% is excreted in feces.[1][2] Steady-state clearance is similar at all ages.[1] |
| Chemical and physical data | |
| Formula | C24H29NO3 |
| Molar mass | 379.500 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Donepezil, sold as the trade name Aricept among others, is a medication used to treat Alzheimer's disease.[5] It appears to result in a small benefit in mental function and ability to function.[6] Use, however, has not been shown to change the progression of the disease.[7] Treatment should be stopped if no benefit is seen.[8] It is taken by mouth.[5]
Common side effects include nausea, trouble sleeping, aggression, diarrhea, feeling tired, and muscle cramps.[5][8] Serious side effects may include abnormal heart rhythms, difficulty emptying urine from the bladder, and seizures.[5] Donepezil is a centrally acting reversible acetylcholinesterase inhibitor and structurally unrelated to other anticholinesterase agents.[5][1]
Donepezil was approved for medical use in the United States in 1996.[5] It is available as a generic medication.[8] In the United Kingdom a typical month's supply costs the NHS about £0.52 as of 2019.[9] The wholesale cost of this amount in the United States is about US$1.38.[10] In 2017, it was the 127th most commonly prescribed medication in the United States, with more than five million prescriptions.[11][12]
Medical uses
Alzheimer's
There is no evidence that donepezil or other similar agents alters the course or progression of Alzheimer's disease. Six-to-twelve-month controlled studies have shown modest benefits in cognition or behavior.[13][14] The UK National Institute for Clinical Excellence (NICE) recommends donepezil as an option in the management of mild to moderate Alzheimer's disease.[15] The person should, however, be reviewed frequently and if there is no significant benefit it should be stopped.[15] In 2006 the U.S. Food and Drug Administration also approved donepezil for treatment of mild, moderate and severe dementia in Alzheimer's disease.[16]
Other
- Lewy body dementia: Some studies have shown benefits of donepezil for the treatment of cognitive and behavioral symptoms in Lewy body dementia.[1]
- Traumatic brain injury: Some research suggests an improvement in memory dysfunction in patients with traumatic brain injury with donepezil use.[1]
- Vascular dementia: Studies have shown that donepezil may improve cognition in patients with vascular dementia but not overall global functioning.[1]
- Dementia associated with Parkinson disease: Some evidence suggests that donepezil can improve cognition, executive function, and global status in Parkinson disease dementia.[1]
Dosage
The defined daily dose is 7.5 mg by mouth.[3]
Side effects
The most common side effects leading to discontinuation are nausea, diarrhea, and vomiting.[17][18] Other side effects included difficulty sleeping, muscle cramps and loss of appetite. Most side effects were observed in patients taking the 23 mg dose compared to 10 mg or lower doses. Side effects are mild and transient in most patients, lasting up to three weeks and usually improved even with continued use.[19][1]
Donepezil, like other cholinesterase inhibitors, can cause nightmares due to enhanced activation of the visual association cortex during REM sleep.[1] Dosing donepezil in the morning can reduce the frequency of nightmares.[1]
Precautions
Donepezil should be used with caution in people with heart disease, cardiac conduction disturbances, chronic obstructive pulmonary disease, asthma, severe cardiac arrhythmia and sick sinus syndrome.[19]
People with peptic ulcer disease or taking NSAIDS should use with caution because increased risk of gastrointestinal bleeding was noted.[19] Slow heart beat and fainting in people with heart problems were also seen. These symptoms may appear more frequent when initiating treatment or increasing the donepezil dose. Although occurrence of seizures is rare, people who have a predisposition to seizures should be treated with caution.[19]
If daily donepezil has suspended for 7 days or less, restarting at the same dose is recommended, while if the suspension lasts longer than 7 days, retitrate from 5 mg daily is suggested.[20][21]
Mechanism of action
Donepezil binds and reversibly inactivates the cholinesterases, thus inhibiting hydrolysis of acetylcholine. This increases acetylcholine concentrations at cholinergic synapses.[1]
The precise mechanism of action of donepezil in patients with Alzheimer's disease is not fully understood. Certainly, Alzheimer's disease involves a substantial loss of the elements of the cholinergic system and it is generally accepted that the symptoms of Alzheimer's disease are related to this cholinergic deficit, particularly in the cerebral cortex and other areas of the brain.[22][23] It is noted that the hippocampal formation plays an important role in the processes of control of attention, memory and learning. Just the severity of the loss of cholinergic neurons of the central nervous system (CNS) has been found to correlate with the severity of cognitive impairment.
In addition to its actions as an acetylcholinesterase inhibitor, donepezil has been found to act as a potent agonist of the σ1 receptor (Ki = 14.6 nM), and has been shown to produce specific antiamnestic effects in animals mainly via this action.[24]
Some noncholinergic mechanisms have also been proposed.[1] Donepezil upregulates the nicotinic receptors in the cortical neurons, adding to neuroprotective property.[1] It inhibits voltage-activated sodium currents reversibly and delays rectifier potassium currents and fast transient potassium currents, although this action is unlikely to contribute to clinical effects.[1]
Stereochemistry
Donepezil medications are racemates.[25]
| Enantiomers | |
|---|---|
-Donepezil_Structural_Formula_V1.svg.png.webp) (R)-Donepezil |
-Donepezil_Structural_Formula_V1.svg.png.webp) (S)-Donepezil |
History
Research leading to the development of donepezil began in 1983 at Eisai, and in 1996, Eisai received approval from the United States Food and Drug Administration (USFDA) for donepezil under the brand Aricept, which it co-marketed with Pfizer.[27] The team at Eisai was led by Hachiro Sugimoto.[28]
As of 2011, Aricept was the world's best-selling Alzheimer's disease treatment.[29] The first generic donepezil became available in November 2010 with the USFDA approval of a formulation prepared by Ranbaxy Labs.[30] In April 2011 a second generic formulation, from Wockhardt, received tentative USFDA marketing approval.[31]
Research
Donepezil has been tested in other cognitive disorders, including Lewy body dementia,[32] and vascular dementia,[33] but it is not currently approved for these indications. Donepezil has also been found to improve sleep apnea in people with Alzheimer's.[34] It also improves gait in people with mild Alzheimer's.[35]
Donepezil has also been studied in people with mild cognitive impairment, schizophrenia, attention deficit disorder, post-coronary artery bypass surgery cognitive impairment,[36] cognitive impairment associated with multiple sclerosis, CADASIL syndrome, and Down syndrome. A three-year National Institutes of Health trial in people with mild cognitive impairment reported donepezil was superior to placebo in delaying rate of progression to dementia during the initial 18 months of the study, but this was not sustained at 36 months.[37] In a secondary analysis, a subgroup of individuals with the apolipoprotein E4 genotype showed sustained benefits with donepezil throughout the study.[38] At this time, though, donepezil is not indicated for prevention of dementia.
ADHD
The addition of donepezil with existing ADHD medications has shown mixed results.[39] In those with Tourette syndrome and ADHD, donepezil may reduce tics while it had no effect on ADHD's symptoms.[39]
Pervasive developmental disorder
Donepezil along with other cholinesterase inhibitors is suggested as having potential for trouble behaviors, irritability, hyperactivity, and difficulty in social communication which are typically seen in those with pervasive developmental disorder, pervasive developmental disorder not otherwise specified, or autism spectrum disorder.[39]
Society and culture
Cost
In the United Kingdom a typical month's supply costs the NHS about £0.52 as of 2019.[9] The wholesale cost of this amount in the United States is about US$1.38.[10] In 2017, it was the 127th most commonly prescribed medication in the United States, with more than five million prescriptions.[11][12]
.svg.png.webp) DonepezilHydrochloride costs (US)
DonepezilHydrochloride costs (US).svg.png.webp) DonepezilHydrochloride prescriptions (US)
DonepezilHydrochloride prescriptions (US)
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Kumar, A; Sharma, S (2020), "article-20656", Donepezil, Treasure Island (FL): StatPearls Publishing, PMID 30020629, archived from the original on 2021-08-28, retrieved 2020-04-12
- 1 2 3 4 5 6 Seltzer, Ben (2005-09-29). "Donepezil: a review". Expert Opinion on Drug Metabolism & Toxicology. Informa Healthcare. 1 (3): 527–36. doi:10.1517/17425255.1.3.527. ISSN 1742-5255. PMID 16863459.
there is a linear relationship between dose and pharmacodynamic effects, measured as red blood cell acetylcholinesterase inhibition and clinical efficacy. Despite being 96% bound to plasma proteins, it has few interactions with other drugs, and the 5-mg dose can be given safely to patients with mild-to-moderate hepatic and renal-disease.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 27 November 2020. Retrieved 9 September 2020.
- ↑ Asiri, Yousif A.; Mostafa, Gamal A.E. (2010). "Donepezil". Profiles of Drug Substances, Excipients and Related Methodology. Vol. 35. Elsevier. pp. 117–50. doi:10.1016/s1871-5125(10)35003-5. ISBN 978-0-12-380884-4. ISSN 1871-5125. PMID 22469221.
Plasma donepezil concentrations decline with a half-life of approximately 70 h. Sex, race, and smoking history have no clinically significant influence on plasma concentrations of donepezil [46–51].
- 1 2 3 4 5 6 "Donepezil Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 26 July 2019. Retrieved 4 February 2019.
- ↑ Birks JS, Harvey RJ (June 2018). "Donepezil for dementia due to Alzheimer's disease". The Cochrane Database of Systematic Reviews. 6: CD001190. doi:10.1002/14651858.CD001190.pub3. PMC 6513124. PMID 29923184.
- ↑ Swedish Council on Health Technology Assessment (June 2008). "Dementia – Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review". PMID 28876770.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 300. ISBN 9780857113382.
- 1 2 BNF76 (2018), p. 300 "Donepezil hydrochloride (Non-proprietary) Donepezil hydrochloride 5 mg Donepezil 5 mg tablets | 28 tablet P £ 59.85 DT = £ 0.52"
- 1 2 "NADAC as of 2019-01-30". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-27. Retrieved 4 February 2019.
0.04978 (per tablet)
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Donepezil Hydrochloride – Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ↑ Steele LS, Glazier RH (April 1999). "Is donepezil effective for treating Alzheimer's disease?". Canadian Family Physician. 45: 917–19. PMC 2328349. PMID 10216789.
- ↑ Fink, Howard A.; Linskens, Eric J.; MacDonald, Roderick; Silverman, Pombie C.; McCarten, J. Riley; Talley, Kristine M.C.; Forte, Mary L.; Desai, Priyanka J.; Nelson, Victoria A.; Miller, Margaret A.; Hemmy, Laura S.; Brasure, Michelle; Taylor, Brent C.; Ng, Weiwen; Ouellette, Jeannine M.; Sheets, Kerry M.; Wilt, Timothy J.; Butler, Mary (19 May 2020). "Benefits and Harms of Prescription Drugs and Supplements for Treatment of Clinical Alzheimer-Type Dementia: A Systematic Review and Meta-analysis". Annals of Internal Medicine. 172 (10): 656–668. doi:10.7326/M19-3887.
- 1 2 "Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease | Guidance and guidelines | NICE". www.nice.org.uk. 23 March 2011. Archived from the original on 31 July 2019. Retrieved 4 February 2019.
- ↑ "FDA Approves Expanded Use of Treatment for Patients With Severe Alzheimer's Disease". FDA. 2006-10-13. Archived from the original on 2009-07-10.
- ↑ "www.accessdata.fda.gov" (PDF). Archived (PDF) from the original on 2015-12-22.
- ↑ Noetzli M, Eap CB (April 2013). "Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer's disease". Clinical Pharmacokinetics. 52 (4): 225–41. doi:10.1007/s40262-013-0038-9. PMID 23408070.
- 1 2 3 4 Aricept (donepezil hydrochloride) package insert. Woodcliff Lake, NJ: Eisai Co., Ltd.; 2010 Nov.
- ↑ "Donepezil: MedlinePlus Drug Information". MedlinePlus. 2019-12-22. Archived from the original on 2019-03-27. Retrieved 2019-12-31.
If you forget to take a dose of donepezil, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one. If you do not take donepezil, for 1 week or longer, you should call your doctor before starting to take this medication again.
- ↑ "Table 4. NHS Guideline" (PDF). p. 6 of 11. Archived from the original (PDF) on 2019-12-31.
Re-titration following AChEI missed doses or planned treatment breaks
- ↑ Davies P, Maloney AJ (December 1976). "Selective loss of central cholinergic neurons in Alzheimer's disease". Lancet. 2 (8000): 1403. doi:10.1016/s0140-6736(76)91936-x. PMID 63862.
- ↑ Kása P, Rakonczay Z, Gulya K (August 1997). "The cholinergic system in Alzheimer's disease". Progress in Neurobiology. 52 (6): 511–35. doi:10.1016/s0301-0082(97)00028-2. PMID 9316159.
- ↑ Maurice T, Su TP (November 2009). "The pharmacology of sigma-1 receptors". Pharmacology & Therapeutics. 124 (2): 195–206. doi:10.1016/j.pharmthera.2009.07.001. PMC 2785038. PMID 19619582.
- ↑ Rote Liste Service GmbH (Hrsg.): Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). Rote Liste Service GmbH, Frankfurt/Main, 2017, Aufl. 57, ISBN 978-3-946057-10-9, S. 178.
- ↑ Proteopedia 1eve
- ↑ Rodrigues Simões MC, Dias Viegas FP, Moreira MS, de Freitas Silva M, Riquiel MM, da Rosa PM, et al. (January 2014). "Donepezil: an important prototype to the design of new drug candidates for Alzheimer's disease". Mini Reviews in Medicinal Chemistry. 14 (1): 2–19. doi:10.2174/1389557513666131119201353. PMID 24251806.
- ↑ "Developed the magic bullet for Alzheimer's disease after overcoming many difficulties (Hachiro Sugimoto)". Chuo University Gakuin Jihou (ChuOnline). Archived from the original on 2020-02-15. Retrieved 2017-01-11.
- ↑ Kanoko Matsuyama (25 April 2011). "Eisai Aricept Patch for Alzheimer's Isn't Ready for Approval". Bloomberg. Archived from the original on 2012-11-05. Retrieved 25 April 2011.
- ↑ "Ranbaxy gets FDA nod for Alzheimer's drug". The Indian Express. New Delhi, India: Indian Express Group. 30 November 2010. IndianExpress.com. Archived from the original on 2 January 2020. Retrieved 25 April 2011.
- ↑ Staff Writer (25 April 2011). "Wockhardt Obtains US FDA Nod For Generic Version Of Aricept Tablets". RTTNews. Archived from the original on 2 January 2020. Retrieved 25 April 2011.
- ↑ Rojas-Fernandez CH (February 2001). "Successful use of donepezil for the treatment of dementia with Lewy bodies". The Annals of Pharmacotherapy. 35 (2): 202–05. doi:10.1345/aph.10192. PMID 11215841.
- ↑ Malouf R, Birks J (2004). Malouf R (ed.). "Donepezil for vascular cognitive impairment". The Cochrane Database of Systematic Reviews (1): CD004395. doi:10.1002/14651858.CD004395.pub2. PMID 14974068.
- ↑ Moraes W, Poyares D, Sukys-Claudino L, Guilleminault C, Tufik S (March 2008). "Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study". Chest. 133 (3): 677–83. doi:10.1378/chest.07-1446. PMID 18198262. Archived from the original on 2013-04-14.
- ↑ Montero-Odasso M, Muir-Hunter SW, Oteng-Amoako A, Gopaul K, Islam A, Borrie M, et al. (2015-01-01). "Donepezil improves gait performance in older adults with mild Alzheimer's disease: a phase II clinical trial". Journal of Alzheimer's Disease. 43 (1): 193–99. doi:10.3233/JAD-140759. PMID 25079803.
- ↑ Doraiswamy PM, Babyak MA, Hennig T, Trivedi R, White WD, Mathew JP, et al. (2007). "Donepezil for cognitive decline following coronary artery bypass surgery: a pilot randomized controlled trial". Psychopharmacology Bulletin. 40 (2): 54–62. PMID 17514186.
- ↑ Jelic V, Kivipelto M, Winblad B (April 2006). "Clinical trials in mild cognitive impairment: lessons for the future". Journal of Neurology, Neurosurgery, and Psychiatry. 77 (4): 429–38. doi:10.1136/jnnp.2005.072926. PMC 2077499. PMID 16306154.
- ↑ Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. (June 2005). "Vitamin E and donepezil for the treatment of mild cognitive impairment". The New England Journal of Medicine. 352 (23): 2379–88. doi:10.1056/nejmoa050151. PMID 15829527.
- 1 2 3 Elbe, Dean (2019). Clinical handbook of psychotropic drugs for children and adolescents. Boston, MA: Hogrefe. pp. 366–69. ISBN 978-1-61676-550-7. OCLC 1063705924.
Further reading
- Brenner, George D.; George M. Brenner (2000). Pharmacology. Philadelphia: W. B. Saunders. ISBN 978-0-7216-7757-6.
- Acting Editor-in-Chief Louise Welbanks. (2000). Compendium of Pharmaceuticals and Specialities, 2000 (25th ed.). Canadian Pharmaceutical Assn. ISBN 978-0-919115-76-7.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Acetylcholinesterase: A gorge-ous enzyme Article describing structure of target enzyme acetylcholinesterase at PDBe Archived 2019-09-18 at the Wayback Machine