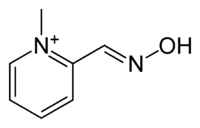
Pralidoxime
 | |
 | |
| Names | |
|---|---|
| Trade names | ATNAA, DuoDote, Protopam, others |
| Other names | 2-pyridine aldoxime methyl chloride, 1-methylpyridine-6-carbaldehyde oxime |
IUPAC name
| |
| Clinical data | |
| Drug class | Oxime[1] |
| Main uses | Organophosphate, anticholinesterase, or nerve agent poisoning[1] |
| Side effects | Blurry vision, headache, sleepiness, nausea, fast heart rate, high blood pressure, pain at the site of injection[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C7H9N2O+ |
| Molar mass | 137.162 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pralidoxime (2-PAM) is a medication used to treat organophosphate, anticholinesterase, and nerve agent poisoning.[1] It is used together with atropine.[1] It is not used for carbamate poisoning.[2] It is given by injection into a vein or muscle.[1]
Common side effects include blurry vision, headache, sleepiness, nausea, fast heart rate, high blood pressure, and pain at the site of injection.[1] It is in the oxime family of medications.[1]
Pralidoxime was approved for medical use in the United States in 1964.[1] In the United States it costs about 90 USD per gram as of 2021.[3] An autoinjector is also available in combination with atropine and diazepam.[4] Some militaries provide these autoinjectors to their soldiers.[4]
Medical uses
Dosage
- Adults: 30 mg/kg (typically 1–2 g), administered by intravenous therapy over 15–30 minutes, repeated 60 minutes later.[4] Higher doses maybe used.[4] It can also be given as a 500 mg/h continuous IV infusion.
- Children: 20–50 mg/kg followed by a maintenance infusion at 5–10 mg/kg/h.
Intravenous infusions can lead to respiratory or cardiac arrest if given too quickly.[5]
Interactions
When atropine and pralidoxime are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, succinylcholine, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning.
Contraindications
There are no known absolute contraindications for the use of pralidoxime. Relative contraindications include known hypersensitivity to the drug and other situations in which the risk of its use clearly outweighs possible benefit.
Mechanism of action
Pralidoxime is typically used in cases of organophosphate poisoning. Organophosphates such as sarin bind to the hydroxy component (the esteric site) of the active site of the acetylcholinesterase enzyme, thereby blocking its activity. Pralidoxime binds to the other half (the unblocked, anionic site) of the active site and then displaces the phosphate from the serine residue. The conjoined poison / antidote then unbinds from the site, and thus regenerates the fully functional enzyme.
Some phosphate-acetylcholinesterase conjugates continue to react after the phosphate docks to the esteric site, evolving into a more recalcitrant state. This process is known as aging. Aged phosphate-acetylcholinesterase conjugate are resistant to antidotes such as pralidoxime. Pralidoxime is often used with atropine (a muscarinic antagonist) to help reduce the parasympathetic effects of organophosphate poisoning. Pralidoxime is only effective in organophosphate toxicity. It has no beneficial effects if the acetylcholinesterase enzyme is carbamylated, as occurs with neostigmine, pyridostigmine, or insecticides such as carbaryl.
Pralidoxime has an important role in reversing paralysis of the respiratory muscles but due to its poor blood–brain barrier penetration, it has little effect on centrally-mediated respiratory depression. Atropine, which is choice of drug to antagonise the muscarinic effects of organophosphates, is administered even before pralidoxime during the treatment of organophosphate poisoning. While the efficacy of atropine has been well-established, clinical experience with pralidoxime has led to widespread doubt about its efficacy in treatment of organophosphorus poisoning.[6]
Chemistry
It It is a white solid.
Synthesis
Pralidoxime, 2-pyridinaldoxime methylchloride, is prepared by treating pyridine-2-carboxaldehyde with hydroxylamine. The resulting pyridine-2-aldoxime is alkylated with methyl iodide giving pralidoxime as the iodide salt.[7][8][9][10]

See also
References
- 1 2 3 4 5 6 7 8 9 "Pralidoxime Monograph for Professionals". Drugs.com. Archived from the original on 18 January 2021. Retrieved 29 October 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1422. ISBN 978-0857114105.
- ↑ "Protopam Chloride Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 28 January 2021. Retrieved 29 October 2021.
- 1 2 3 4 Gupta, R; Parmar, M (January 2021). "Pralidoxime". PMID 32644334.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Baxter Healthcare Corporation 2006, Protopam Prescribing Information
- ↑ Banerjee I, Tripathi SK, Roy AS (2014). "Efficacy of pralidoxime in organophosphorus poisoning: revisiting the controversy in Indian setting". Journal of Postgraduate Medicine. 60 (1): 27–30. doi:10.4103/0022-3859.128803. PMID 24625936.
- ↑ US 2816113, Nachmansonn E, Ginsburg S, published 1957
- ↑ US 3123613, Black LP, published 1964
- ↑ US 3140289, Easterday DE, Kondritzer AA, published 1964
- ↑ US 3155674, McDowell WB, published 1964
External links
| Identifiers: |
|---|