Flumazenil
 | |
 | |
| Names | |
|---|---|
| Trade names | Anexate, Lanexat, Mazicon, Romazicon |
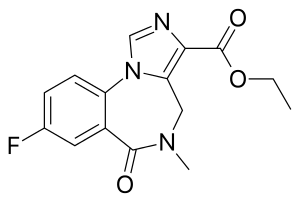
| Other names | Flumazepil, Ro 15-1788, ethyl 8-fluoro- 5,6-dihydro- 5-methyl- 6-oxo- 4H- imidazo [1,5-a] [1,4] benzodiazepine- 3-carboxylate |
IUPAC name
| |
| Clinical data | |
| Main uses | Reverse effects of benzodiazepines[1] |
| Side effects | Sweating, blurry vision, headache, dizziness, seizures[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | IV |
| Onset of action | Within 2 min[2] |
| Duration of action | 40 to 90 min[3] |
| Typical dose | 0.2 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 7–15 min (initial) 20–30 min (brain) 40–80 min (terminal) |
| Excretion | Urine 90–95% Feces 5–10% |
| Chemical and physical data | |
| Formula | C15H14FN3O3 |
| Molar mass | 303.293 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Flumazenil, sold under the brand name Anexate, is a medication used to reverse the effects of benzodiazepines.[1] Routine use is not recommended in those with a decreased level of consciousness.[4] It is given by injection into a vein.[1] Effects begin within 2 minutes and may last for up to 90 minutes.[2][3]
Common side effects include sweating, blurry vision, headache, and dizziness.[2] Other side effects may include seizures and benzodiazepine withdrawal, especially in people with benzodiazepine dependence.[2] Panic attacks may also occur.[2] Safety in pregnancy is unclear.[5] It works by blocking benzodiazepines at the GABA receptor.[2]
Flumazenil was characterized in 1981, and approved for medical use in the United States in 1991.[6][2] It is available as a generic medication.[1] In the United Kingdom 0.5 mg cost the NHS about £13 as of 2021.[1] This amount in the United States is about 10 USD.[7]
Medical uses
Benzodiazepine overdose
Flumazenil has been used in people who become excessively drowsy after use of benzodiazepines for either diagnostic or treatment purposes.[8] It is not recommended for routine use in those with a decreased level of consciousness.[4] It is also not of use in cardiac arrest due to benzodiazepines.[9]
It has been used as an antidote in the treatment of benzodiazepine overdoses.[8] There are many complications that must be taken into consideration when used in the acute care setting.[8] These include lowered seizure threshold, agitation, and anxiousness.
Flumazenil's short half-life may require multiple doses. Because of the potential risks of withdrawal symptoms and the drug's short half-life, people must be carefully monitored to prevent recurrence of symptoms.
Flumazenil has also been effectively used to treat overdoses of non-benzodiazepine hypnotics, such as zolpidem, zaleplon and zopiclone.[10]
Benzodiazepine dependence
These is tentative evidence to support the use of low dose flumazenil over 4 days to treat benzodiazepine dependence.[11] However concerns regarding panic attacks with its use were seen.[12]
Cirrhosis
The drug has also been used in hepatic encephalopathy. It may have beneficial short‐term effects in people with cirrhosis, but there is no evidence for long-term benefits.[13]
Dosage
The onset of action is rapid, and effects are usually seen within one to two minutes. The peak effect is seen at six to ten minutes. The recommended dose for adults is 200 μg every 1–2 minutes until the effect is seen, up to a maximum of 3 mg per hour. It is available as a clear, colourless solution for intravenous injection, containing 500 μg in 5 mL.
Side effects
Individuals who are dependent on benzodiazepines may develop benzodiazepine withdrawal symptoms, including seizure, upon being given flumazenil.
Pharmacology
Flumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on the central nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex.[14] It also exhibits weak partial agonism of GABAA receptor complexes that contain α6-type monomers; the clinical relevance of this is unknown.[15]
Flumazenil does not antagonize all of the central nervous system effects of drugs affecting GABA-ergic neurons by means other than the benzodiazepine receptor (including ethanol, barbiturates, and most anesthetics) and does not reverse the effects of opioids. It will however antagonize the action of non-benzodiazepine z-drugs, such as zolpidem and zopiclone, because they act via the benzodiazepine site of the GABA receptor[16] - it has been used to successfully treat z-drug overdose.[16][17][18]
It is metabolised by the liver to inactive compounds which are excreted in the urine.
Pharmacodynamics
Intravenous flumazenil has been shown to antagonize sedation, impairment of recall, psychomotor impairment and ventilatory depression produced by benzodiazepines in healthy human volunteers.
The duration and degree of reversal of sedative benzodiazepine effects are related to the dose and plasma concentrations of flumazenil.
Society and culture
Availability
Flumazenil is sold under a wide variety of brand names worldwide like Anexate, Lanexat, Mazicon, Romazicon. In India it is manufactured by Roche Bangladesh Pharmaceuticals and USAN Pharmaceuticals.
Research
Radiolabeled with the radioactive isotope carbon-11, flumazenil may be used as a radioligand in neuroimaging with positron emission tomography to visualize the distribution of GABAA receptors in the human brain.[19]
References
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1422. ISBN 978-0857114105.
- 1 2 3 4 5 6 7 8 "Flumazenil Monograph for Professionals". Drugs.com. Archived from the original on 30 September 2020. Retrieved 11 December 2021.
- 1 2 3 Wilson, William C.; Grande, Christopher M.; Hoyt, David B. (5 February 2007). Trauma: Critical Care. CRC Press. p. 85. ISBN 978-1-4200-1684-0. Archived from the original on 11 December 2021. Retrieved 11 December 2021.
- 1 2 Wood, Lawrence D. H.; Hall, Jesse B.; Schmidt, Gregory D. 1952 (2005). Principles of critical care. McGraw-Hill Professional. ISBN 978-0-07-141640-5. Archived from the original on 2020-12-16. Retrieved 2021-06-07.
- ↑ "Flumazenil (Romazicon) Use During Pregnancy". Drugs.com. Archived from the original on 3 December 2020. Retrieved 11 December 2021.
- ↑ Whitwam, James. G. (1988). "Flumazenil: a benzodiazepine antagonist". BMJ. 297 (6655): 999–1000. doi:10.1136/bmj.297.6655.999. PMC 1834756. PMID 2903780.
- ↑ "Flumazenil Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 11 December 2021.
- 1 2 3 Goldfrank, Lewis R. (2002). Goldfrank's toxicologic emergencies. New York: McGraw-Hill Medical Publ. Division. ISBN 978-0-07-136001-2. Archived from the original on 2020-12-16. Retrieved 2021-06-07.
- ↑ Lavonas, EJ; Akpunonu, PD; Arens, AM; Babu, KM; Cao, D; Hoffman, RS; Hoyte, CO; Mazer-Amirshahi, ME; Stolbach, A; St-Onge, M; Thompson, TM; Wang, GS; Hoover, AV; Drennan, IR; American Heart, Association (17 October 2023). "2023 American Heart Association Focused Update on the Management of Patients With Cardiac Arrest or Life-Threatening Toxicity Due to Poisoning: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 148 (16): e149–e184. doi:10.1161/CIR.0000000000001161. PMID 37721023.
- ↑ Nelson, Lewis H.; Flomenbaum, Neal; Goldfrank, Lewis R.; Hoffman, Robert Louis; Howland, Mary Deems; Neal A. Lewin (2006). Goldfrank's toxicologic emergencies. New York: McGraw-Hill, Medical Pub. Division. ISBN 978-0-07-147914-1.
- ↑ Hood, SD; Norman, A; Hince, DA; Melichar, JK; Hulse, GK (February 2014). "Benzodiazepine dependence and its treatment with low dose flumazenil". British journal of clinical pharmacology. 77 (2): 285–94. doi:10.1111/bcp.12023. PMID 23126253.
- ↑ Baandrup, L; Ebdrup, BH; Rasmussen, JØ; Lindschou, J; Gluud, C; Glenthøj, BY (15 March 2018). "Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users". The Cochrane database of systematic reviews. 3: CD011481. doi:10.1002/14651858.CD011481.pub2. PMID 29543325.
- ↑ Goh, ET; Andersen, ML; Morgan, MY; Gluud, LL (10 August 2017). "Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy" (PDF). Cochrane Database of Systematic Reviews. 8: CD002798. doi:10.1002/14651858.CD002798.pub4. PMC 6483298. PMID 28796283. Archived (PDF) from the original on 26 April 2019. Retrieved 7 June 2021.
- ↑ Hood, Sean David; Norman, Amanda; Hince, Dana Adelle; Melichar, Jan Krzysztof; Hulse, Gary Kenneth (February 2014). "Benzodiazepine dependence and its treatment with low dose flumazenil". British Journal of Clinical Pharmacology. 77 (2): 285–294. doi:10.1111/bcp.12023. ISSN 1365-2125. PMC 4014019. PMID 23126253.
- ↑ Hadingham, K. L.; Garrett, E. M.; Wafford, K. A.; Bain, C.; Heavens, R. P.; Sirinathsinghji, D. J.; Whiting, P. J. (February 1996). "Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors". Molecular Pharmacology. 49 (2): 253–259. ISSN 0026-895X. PMID 8632757.
- 1 2 Gunja, Naren (June 2013). "The clinical and forensic toxicology of Z-drugs". Journal of Medical Toxicology. 9 (2): 155–162. doi:10.1007/s13181-013-0292-0. ISSN 1937-6995. PMC 3657020. PMID 23404347.
- ↑ Thornton, Stephen L.; Negus, Elezer; Carstairs, Shaun D. (November 2013). "Pediatric zolpidem ingestion demonstrating zero-order kinetics treated with flumazenil". Pediatric Emergency Care. 29 (11): 1204–1206. doi:10.1097/PEC.0b013e3182aa139c. ISSN 1535-1815. PMID 24196090. S2CID 34655918.
- ↑ Lheureux, P.; Debailleul, G.; De Witte, O.; Askenasi, R. (March 1990). "Zolpidem intoxication mimicking narcotic overdose: response to flumazenil". Human & Experimental Toxicology. 9 (2): 105–107. doi:10.1177/096032719000900209. ISSN 0960-3271. PMID 2111156. S2CID 34525063.
- ↑ Alexander Hammers; Matthias J. Koepp; Mark P. Richardson; Rene Hurlemann; David J. Brooks & John S. Duncan (June 2003). "Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients". Brain. 126 (Pt 6): 1300–1308. doi:10.1093/brain/awg138. PMID 12764053.
External links
| Identifiers: |
|---|
- Flumazenil drug label/data at DailyMed from U.S. National Library of Medicine, National Institutes of Health.