CGP-7930
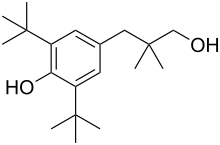 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H32O2 |
| Molar mass | 292.463 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
CGP-7930 is a compound used in scientific research which acts as a positive allosteric modulator at the GABAB receptor.[1][2][3][4] It has anxiolytic effects in animal studies,[5][6] and has a synergistic effect with GABAB agonists such as baclofen and GHB,[7][8] as well as reducing self-administration of alcoholic drinks and cocaine.[9][10]
References
- ↑ Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, et al. (November 2001). "Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501". Molecular Pharmacology. 60 (5): 963–71. PMID 11641424.
- ↑ Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prézeau L (July 2004). "The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor". The Journal of Biological Chemistry. 279 (28): 29085–91. doi:10.1074/jbc.M400930200. PMID 15126507.
- ↑ Chen Y, Menendez-Roche N, Sher E (June 2006). "Differential modulation by the GABAB receptor allosteric potentiator 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)-phenol (CGP7930) of synaptic transmission in the rat hippocampal CA1 area". The Journal of Pharmacology and Experimental Therapeutics. 317 (3): 1170–7. doi:10.1124/jpet.105.099176. PMID 16507713.
- ↑ Adams CL, Lawrence AJ. "CGP7930: a positive allosteric modulator of the GABAB receptor". CNS Drug Reviews. 13 (3): 308–16. doi:10.1111/j.1527-3458.2007.00021.x. PMC 6494120. PMID 17894647.
- ↑ Frankowska M, Filip M, Przegaliński E. "Effects of GABAB receptor ligands in animal tests of depression and anxiety". Pharmacological Reports. 59 (6): 645–55. PMID 18195453.
- ↑ Jacobson LH, Cryan JF (April 2008). "Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents". Neuropharmacology. 54 (5): 854–62. doi:10.1016/j.neuropharm.2008.01.004. PMID 18328507.
- ↑ Carai MA, Colombo G, Froestl W, Gessa GL (November 2004). "In vivo effectiveness of CGP7930, a positive allosteric modulator of the GABAB receptor". European Journal of Pharmacology. 504 (3): 213–6. doi:10.1016/j.ejphar.2004.10.008. PMID 15541424.
- ↑ Parker DA, Marino V, Ong J, Puspawati NM, Prager RH (September 2008). "The CGP7930 analogue 2,6-di-tert-butyl-4-(3-hydroxy-2-spiropentylpropyl)-phenol (BSPP) potentiates baclofen action at GABA(B) autoreceptors". Clinical and Experimental Pharmacology & Physiology. 35 (9): 1113–5. doi:10.1111/j.1440-1681.2008.04948.x. PMID 18430050.
- ↑ Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, et al. (April 2006). "The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats". Neuropharmacology. 50 (5): 632–9. doi:10.1016/j.neuropharm.2005.11.011. PMID 16406445.
- ↑ Filip M, Frankowska M, Przegaliński E (November 2007). "Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination". European Journal of Pharmacology. 574 (2–3): 148–57. doi:10.1016/j.ejphar.2007.07.048. PMID 17698060.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.