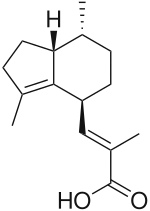
Valerenic acid
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-[(4S,7R,7aR)-3,7-Dimethyl-2,4,5,6,7,7a-hexahydro-1H-inden-4-yl]-2-methylprop-2-enoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.112.154 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H22O2 |
| Molar mass | 234.334 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Valerenic acid is a sesquiterpenoid constituent of the essential oil of the valerian plant.
Valerian is used as a herbal sedative which may be helpful in the treatment of insomnia. Valerenic acid may be at least partly responsible for the sedative effects.[1]
Valerenic acid acts as a subtype-selective GABAA receptor positive allosteric modulator via a binding site in the transmembrane domain at the β+α− interface.[2] At receptors expressed in Xenopus oocytes (frog eggs) it was shown that only assemblies incorporating β2 or β3 subunits were stimulated by valerenic acid. A study in mice demonstrated that a single amino acid substitution (N265M) in the β3 subunit severely decreases the anxiolytic effect. Modulation of ion channel action was not significantly dependent on incorporation of α1, α2, α3 or α5 subunits.[3]
At the 5-HT5A receptor valerenic acid acts as a partial agonist. This serotonin receptor subtype is distributed in the suprachiasmatic nucleus, a tiny brain region implicated in the sleep-wake cycle.[4]
A study in 2006 found valerian extract as well as valerenic acid to inhibit NF-κB, a protein complex that controls the transcription of DNA, in HeLa (cultured human cancer) cells. This was measured with the IL-6 / Luc (Interleukin-6 luciferase) assay as a measurement tool. The study mentioned that such inhibition may be connected to the reported anti-inflammatory action of the valerian plant.[5]
References
- ↑ Yuan, C. S.; Mehendale, S.; Xiao, Y.; Aung, H. H.; Xie, J. T.; Ang-Lee, M. K. (2004). "The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity" (PDF). Anesthesia and Analgesia. 98 (2): 353–358. CiteSeerX 10.1.1.323.5518. doi:10.1213/01.ANE.0000096189.70405.A5. PMID 14742369. S2CID 14526474.
- ↑ Luger D, Poli G, Wieder M, Stadler M, Ke S, Ernst M, Hohaus A, Linder T, Seidel T, Langer T, Khom S, Hering S (2015). "Identification of the putative binding pocket of valerenic acid on GABAA receptors using docking studies and site-directed mutagenesis". Br. J. Pharmacol. 172 (22): 5403–13. doi:10.1111/bph.13329. PMC 4988470. PMID 26375408.
- ↑ Khom, S.; Baburin, I.; Timin, E.; Hohaus, A.; Trauner, G.; Kopp, B.; Hering, S. (2007). "Valerenic acid potentiates and inhibits GABAA receptors: Molecular mechanism and subunit specificity". Neuropharmacology. 53 (1): 178–187. doi:10.1016/j.neuropharm.2007.04.018. PMID 17585957. S2CID 7613630.
- ↑ Dietz, B.; Mahady, G.; Pauli, G.; Farnsworth, N. (2005). "Valerian extract and valerenic acid are partial agonists of the 5-HT receptor in vitro". Molecular Brain Research. 138 (2): 191–197. doi:10.1016/j.molbrainres.2005.04.009. PMC 5805132. PMID 15921820.
- ↑ Jacobo-Herrera, N. J.; Vartiainen, N.; Bremner, P.; Gibbons, S.; Koistinaho, J.; Heinrich, M. (2006). "F-κB modulators from Valeriana officinalis". Phytotherapy Research. 20 (10): 917–919. doi:10.1002/ptr.1972. PMID 16909443. S2CID 44352988.