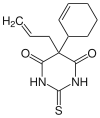
Thialbarbital
 | |
| Clinical data | |
|---|---|
| Other names | Kemithal, 5-(1-cyclohex-2-enyl)-5-prop-2-enyl-2-sulfanylidene-1,3-diazinane-4,6-dione |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| ECHA InfoCard | 100.006.720 |
| Chemical and physical data | |
| Formula | C13H16N2O2S |
| Molar mass | 264.34 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Thialbarbital (Intranarcon) is a barbiturate derivative invented in the 1960s. It has sedative effects, and was used primarily for induction in surgical anaesthesia.[1] Thialbarbital is short acting and has less of a tendency to induce respiratory depression than other barbiturate derivatives such as pentobarbital.[2]
Synthesis
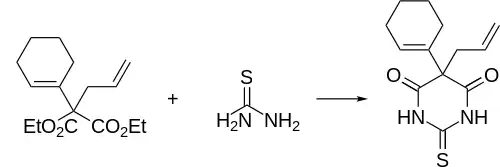
Thialbarbital synthesis: Volwiler, Tabern, U.S. Patent 2,153,730 (1939 to Abbott)
See also
References
- ↑ Golovchinsky VB, Plehotkina SI (July 1971). "Difference in the sensitivity of the cerebral cortex and midbrain reticular formation to the action of diethylether and thialbarbital". Brain Research. 30 (1): 37–47. doi:10.1016/0006-8993(71)90004-7. PMID 5092630.
- ↑ Bercovitz AB, Godke RA, Biellier HV, Short CE (March 1975). "Surgical anesthesia in turkeys with thialbarbital sodium". American Journal of Veterinary Research. 36 (3): 301–2. PMID 1115429.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.