Ganaxolone
 | |
| Names | |
|---|---|
| Trade names | Ztalmy |
| Other names | GNX; CCD-1042; 3β-Methyl-5α-pregnan-3α-ol-20-one; 3α-Hydroxy-3β-methyl-5α-pregnan-20-one |
IUPAC name
| |
| Clinical data | |
| Drug class | Neurosteroid |
| Main uses | Seizures in cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD)[1] |
| Side effects | Sleepiness, fever, increased saliva, seasonal allergies[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
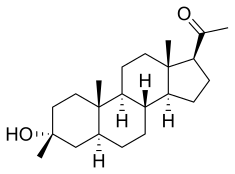
| Formula | C22H36O2 |
| Molar mass | 332.528 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ganaxolone, sold under the brand name Ztalmy, is a medication used to treat seizures in people with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD).[1] It may be used in those who are at least 2 years old.[1] It is taken by mouth.[1]
Common side effects include sleepiness, fever, increased saliva, and seasonal allergies.[1] Other side effects may include suicidal thoughts.[1] It is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator.[1]
Ganaxolone was approved for medical use in the United States in 2022.[1] It is an orphan drug in Europe and not approved in the United Kingdom as of 2022.[2] In the United States it costs about 133,000 USD for a person who is around 4.5 years old as of 2022.[2]
Medical uses
Dosage
In those who weight less than 28 kg it is started at 6 mg/kg three times per day and may be increased up to 21 mg/kg three times per day.[1] In those over 28 kg it is started at 150 mg three times per day and may be increased up to 600 mg three times per day.[1]
Pharmacology
Mechanism of action
The exact mechanism of action for ganaxolone is unknown; however, results from animal studies suggest that it acts by blocking seizure propagation and elevating seizure thresholds.[3][4]
Ganaxolone is thought to modulate both synaptic and extrasynaptic GABAA receptors to normalize over-excited neurons.[5] Ganaxolone's activation of the extrasynaptic receptor is an additional mechanism that provides stabilizing effects that potentially differentiates it from other drugs that increase GABA signaling.[5]
Ganaxolone binds to allosteric sites of the GABAA receptor to modulate and open the chloride ion channel, resulting in a hyperpolarization of the neuron.[5] This causes an inhibitory effect on neurotransmission, reducing the chance of a successful action potential (depolarization) from occurring.[5][3][4]
It is unknown if ganaxolone possesses significant hormonal activity in vivo, with a 2020 study finding evidence of in vitro binding to the membrane progesterone receptor.[6]
Chemistry
Ganaxolone is a analog of the neuroactive steroid allopregnanolone which possesses no known hormonal activity and, instead, is thought to primarily function by binding to GABAa receptors as a positive allosteric modulator.[7]
Other pregnane neurosteroids include alfadolone, alfaxolone, allopregnanolone (brexanolone), hydroxydione, minaxolone, pregnanolone (eltanolone), and renanolone.
Research
Ganaxolone is being investigated for potential medical use in the treatment of epilepsy. It is well tolerated in human trials, with the most commonly reported side effects being somnolence (sleepiness), dizziness, and fatigue.[8] Trials in adults with focal onset seizures and in children with infantile spasms have recently been completed.[9][10] There are ongoing studies in patients with focal onset seizures, PCDH19 pediatric epilepsy, and behaviors in Fragile X syndrome.[9][10]
Ganaxolone has been shown to protect against seizures in animal models,[3][4] and to act a positive allosteric modulator of the GABAA receptor.[5][11]
In 2015, the MIND Institute at the University of California, Davis, announced that it was conducting, in collaboration with Marinus Pharmaceuticals, a randomized, placebo-controlled, Phase 2 clinical trial evaluating the effect of ganaxolone on behaviors associated with Fragile X syndrome in children and adolescents.[12][13][14]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "DailyMed - ZTALMY- ganaxolone suspension". dailymed.nlm.nih.gov. Archived from the original on 12 December 2022. Retrieved 11 December 2022.
- 1 2 "Ganaxolone". SPS - Specialist Pharmacy Service. 17 September 2020. Archived from the original on 25 September 2021. Retrieved 11 December 2022.
- 1 2 3 Kaminski RM, Livingood MR, Rogawski MA (July 2004). "Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice". Epilepsia. 45 (7): 864–7. doi:10.1111/j.0013-9580.2004.04504.x. PMID 15230714. S2CID 21974013.
- 1 2 3 Reddy DS, Rogawski MA (May 2010). "Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model". Epilepsy Research. 89 (2–3): 254–60. doi:10.1016/j.eplepsyres.2010.01.009. PMC 2854307. PMID 20172694.
- 1 2 3 4 5 Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW (March 1997). "Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor". The Journal of Pharmacology and Experimental Therapeutics. 280 (3): 1284–95. PMID 9067315.
- ↑ Thomas, Peter; Pang, Yefei (Jun 24, 2020). "Anti-apoptotic Actions of Allopregnanolone and Ganaxolone Mediated Through Membrane Progesterone Receptors (PAQRs) in Neuronal Cells". Front. Endocrinol. 11 (417): 417. doi:10.3389/fendo.2020.00417. PMC 7331777. PMID 32670200.
- ↑ "PubChem compound summary for ganaxolone". PubChem databade. National Library of Medicine (National Center for Biotechnology Information). Archived from the original on 10 December 2022. Retrieved 6 August 2022.
- ↑ Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA (September 1997). "Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity". Epilepsia. 38 (9): 1026–31. doi:10.1111/j.1528-1157.1997.tb01486.x. PMID 9579942. S2CID 27584114.
- 1 2 Nohria V, Giller E (Jan 2007). "Ganaxolone". Neurotherapeutics. 4 (1): 102–5. doi:10.1016/j.nurt.2006.11.003. PMC 7479704. PMID 17199022.
- 1 2 Pieribone VA, Tsai J, Soufflet C, Rey E, Shaw K, Giller E, Dulac O (October 2007). "Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy". Epilepsia. 48 (10): 1870–4. doi:10.1111/j.1528-1167.2007.01182.x. PMID 17634060. S2CID 24656918.
- ↑ Reddy DS, Rogawski MA (December 2000). "Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself". The Journal of Pharmacology and Experimental Therapeutics. 295 (3): 1241–8. PMID 11082461.
- ↑ "Fragile X Research and Treatment Center: Clinical Research Studies" (PDF). UC Davis MIND Institute. 10 February 2015. Archived from the original (PDF) on 5 June 2015. Retrieved 27 January 2016.
- ↑ "Ganaxolone Treatment in Children With Fragile X Syndrome". Clinicaltrials.gov. 7 November 2012. Archived from the original on 10 December 2022. Retrieved 27 January 2016.
- ↑ "UC Davis Health System. UC Davis researchers win $3 million grant from U.S. Congress to study fragile X" (Press release). UC Davis Health System. 8 February 2011. Archived from the original on 3 February 2016. Retrieved 27 January 2016.
External links
| External sites: |
|
|---|---|
| Identifiers: |