Methoxyflurane
 | |
 | |
| Names | |
|---|---|
| Trade names | Penthrane, Metofane, Penthrox, others |
| Other names | 2,2-dichloro-1,1-difluoroethyl methyl ether |
IUPAC name
| |
| Clinical data | |
| Drug class | Volatile anesthetic |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Inhaled |
| Onset of action | Rapid[1] |
| Duration of action | Several minutes[1] |
| Defined daily dose | not established[2] |
| External links | |
| AHFS/Drugs.com | Consumer Drug Information |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | 70% |
| Chemical and physical data | |
| Formula | C3H4Cl2F2O |
| Molar mass | 164.96 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Methoxyflurane, marketed as Penthrox among others, is an inhaled medication primarily used to reduce pain following trauma.[3][4] It may also be used for short episodes of pain as a result of medical procedures.[1] Onset of pain relief is rapid and of a short duration.[1] Use is only recommended with direct medical supervision.[3]
Common side effects include anxiety, headache, sleepiness, cough, and nausea.[3] Serious side effects may include kidney problems, liver problems, low blood pressure, and malignant hyperthermia.[3][1] It is unclear if it is safe in either pregnancy or breastfeeding.[3] It is only recommended in those who have a normal level of consciousness and stable blood pressure and heart rate.[1] It is classified as a volatile anaesthetic.[1]
It was first made in 1948 by William T. Miller and came into medical use in the 1960s.[5] It was used as a general anesthetic from its introduction in 1960 until the late 1970s.[6] In 1999, the manufacturer discontinued methoxyflurane in the United States, and in 2005 the Food and Drug Administration withdrew it from the market.[6] It is still used in New Zealand, Australia, Ireland, and the United Kingdom for pain.[7][1][8][3][9] In the United Kingdom it costs the NHS about £18 per dose as of 2018.[3]
Medical use
Methoxyflurane is used for relief of moderate or severe pain as a result of trauma.[4][3] It may also be used for short episodes of pain as a result of procedures.[1]
Each dose lasts approximately 30 minutes.[10] Pain relief begins after 6–8 breaths and continues for several minutes after stopping inhalation.[11] The maximum recommended dose is 6 milliliters per day or 15 milliliters per week because of the risk of kidney problems, and it is not recommended to be used on consecutive days.[1] Despite the potential for kidney problems when used at anesthetic doses, no significant adverse effects have been reported when it is used at the lower doses (up to 6 milliliters) used for pain relief.[12][13][14] Due to the risk of kidney toxicity, methoxyflurane is contraindicated in people with pre-existing kidney disease or diabetes mellitus, and is not recommended to be administered in conjunction with tetracyclines or other potentially nephrotoxic or enzyme-inducing drugs.[13]
It is self-administered to children and adults using a hand-held inhaler device.[15][12][16][13] A non-opioid alternative to morphine, it is also easier to use than nitrous oxide.[1] A portable, disposable, single-use inhaler device, along with a single 3 milliliter brown glass vial of methoxyflurane allows people who are conscious and hemodynamically stable (including children over the age of 5 years) to self-administer the medication, under supervision.[1]
Dosage
The defined daily dose is not established[2]
Side effects
The consensus is that the use of methoxyflurane should be restricted only to healthy individuals, in situations where it offers specific advantages and even then, only at dosages less than 2.5 MAC hours.[17][18] The National Institute for Occupational Safety and Health maintains a recommended exposure limit for methoxyflurane as waste anesthetic gas of 2 ppm (13.5 mg/m3) over 60 minutes.[19]
Kidney
The first report of nephrotoxicity appeared in 1964, when Paddock and colleagues reported three cases of acute kidney injury, two of whom were found to have calcium oxalate crystals in the renal tubules at autopsy.[20] In 1966, Crandell and colleagues reported a series in which 17/95 (18%) of patients developed an unusual type of nephropathy after operations in which methoxyflurane was used as a general anesthetic. This particular type of chronic kidney disease was characterized by vasopressin-resistant high-output kidney failure (production of large volumes of poorly concentrated urine) with a negative fluid balance, pronounced weight loss, elevation of serum sodium, chloride, osmolality and blood urea nitrogen. The urine of these patients was of a relatively fixed specific gravity and an osmolality very similar to that of the serum. Furthermore, the high urine output persisted a challenge test of fluid deprivation. Most cases resolved within 2–3 weeks, but evidence of renal dysfunction persisted for more than one year in 3 of these 17 cases (18%), and more than two years in one case (6%).[21]
Compared with halothane, methoxyflurane produces dose-dependent and abnormalities in kidney function. The authors showed that subclinical nephrotoxicity occurred following methoxyflurane at minimum alveolar concentration (MAC) for 2.5 to 3 hours (2.5 to 3 MAC hours), while overt toxicity was present in all patients at dosages greater than five MAC hours.[17] This study provided a model that would be used for the assessment of the nephrotoxicity of volatile anesthetics for the next two decades.[22] Furthermore, the concurrent use of tetracyclines and methoxyflurane has been reported to result in fatal renal toxicity.[23]
Liver
Reports of severe and even fatal hepatotoxicity related to the use of methoxyflurane began to appear in 1966.
Mechanism
The biodegradation of methoxyflurane begins immediately. The kidney and liver toxicity observed after anesthetic doses is attributable to one or more metabolites produced by O-demethylation of methoxyflurane. Products of this catabolic process include methoxyfluoroacetic acid (MFAA), dichloroacetic acid (DCAA), and inorganic fluoride.[18] Methoxyflurane nephrotoxicity is dose dependent[21][24][25] and irreversible, resulting from O-demethylation of methoxyflurane to fluoride and DCAA.[1] It is not entirely clear whether the fluoride itself is toxic—it may simply be a surrogate measure for some other toxic metabolite.[26] The concurrent formation of inorganic fluoride and DCAA is unique to methoxyflurane biotransformation compared with other volatile anesthetics, and this combination is more toxic than fluoride alone. This may explain why fluoride formation from methoxyflurane is associated with nephrotoxicity, while fluoride formation from other volatile anesthetics (such as enflurane and sevoflurane) is not.[27]
Pharmacokinetics
Methoxyflurane has a very high lipid solubility (oil:gas partition coefficient of around 950), which gives it very slow pharmacokinetics (induction and emergence characteristics); this being undesirable for routine application in the clinical setting. Initial studies performed in 1961 revealed that in unpremedicated healthy individuals, induction of general anesthesia with methoxyflurane-oxygen alone or with nitrous oxide was difficult or even impossible using the vaporizers available at that time. It was found to be necessary to administer an intravenous anesthetic agent such as sodium thiopental to ensure a smooth and rapid induction. It was further found that after thiopental induction, it was necessary to administer nitrous oxide for at least ten minutes before a sufficient amount of methoxyflurane could accumulate in the bloodstream to ensure an adequate level of anesthesia. This was despite using high flow (liters/minute) of nitrous oxide and oxygen, and with the vaporizers delivering the maximum possible concentration of methoxyflurane.[28]
Similar to its induction pharmacokinetics, methoxyflurane has very slow and somewhat unpredictable emergence characteristics. During initial clinical studies in 1961, the average time to emergence after discontinuation of methoxyflurane was 59 minutes after administration of methoxyflurane for an average duration of 87 minutes. The longest time to emergence was 285 minutes, after 165 minutes of methoxyflurane administration.[28]
Pharmacodynamics
Heart
The effects of methoxyflurane on the circulatory system resemble those of diethyl ether.[29] In dogs, methoxyflurane anesthesia causes a moderate decrease in blood pressure with minimal changes in heart rate, and no significant effect on blood sugar, epinephrine, or norepinephrine. Bleeding and increased arterial partial pressure of carbon dioxide (PaCO2) both induce further decreases in blood pressure, as well as increases in blood glucose, epinephrine and norepinephrine.[30] In humans, methoxyflurane produces some decrease in blood pressure, but cardiac output, stroke volume, and total peripheral resistance are only minimally depressed. Its effect on the pulmonary circulation is negligible, and it does not predispose the heart to cardiac dysrhythmias.[28][31][32][33]
Lungs
Unlike diethyl ether, methoxyflurane is a significant respiratory depressant. In dogs, methoxyflurane causes a dose-dependent decrease in respiratory rate and a marked decrease in respiratory minute volume, with a relatively mild decrease in tidal volume. In humans, methoxyflurane causes a dose-dependent decrease in tidal volume and minute volume, with respiratory rate relatively constant.[29] The net effect of these changes is profound respiratory depression, as evidenced by CO2 retention with a concomitant decrease in arterial pH (this is referred to as a respiratory acidosis) when anesthetized subjects are allowed to breathe spontaneously for any length of time.[28]
Gastrointestinal
In a series of 500 consecutive pregnant people, vomiting occurred in 12 (4.8%) during or after administration of methoxyflurane anesthesia. These findings compared favorably with those reported for cyclopropane (42%), trichloroethylene (28%), and halothane (4.6%).[34] In another study of 645 obstetric patients, Romagnoli and Korman observed 8 cases (1.2%) of postoperative vomiting, one of whom had been retching before the administration of the drug.[35]
Pain
Although the high blood solubility of methoxyflurane is often undesirable, this property makes it useful in certain situations—it persists in the lipid compartment of the body for a long time, providing sedation and analgesia well into the postoperative period.[36][29] There is substantial data to indicate that methoxyflurane is an effective analgesic and sedative agent at subanesthetic doses.[15][12][37][38][39][40][41][42][43][44][45][46][47] Supervised self-administration of methoxyflurane in children and adults can briefly lead to deep sedation,[12] and it has been used as a patient controlled analgesic for painful procedures in children in hospital emergency departments.[16] During childbirth, administration of methoxyflurane produces significantly better analgesia, less psychomotor agitation, and only slightly more somnolence than trichloroethylene.[39]
Central nervous system
Similar to other inhalational anesthetics, the exact mechanism of action is not clearly defined and likely involves multiple molecular targets in the brain and spinal cord.[48][49] Methoxyflurane is a positive allosteric modulator of GABAA and glycine receptors as demonstrated in electrophysiology studies.[50][51] This mechanism is shared with alcohols that produce general anesthesia.[52]
Chemical properties
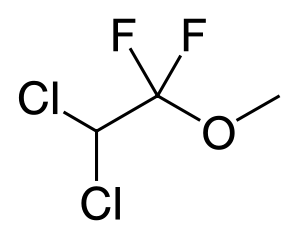
With a molecular formula of C3H4Cl2F2O and a condensed structural formula of CHCl2CF2OCH3, the International Union of Pure and Applied Chemistry (IUPAC) name for methoxyflurane is 2,2-dichloro-1,1-difluoro-1-methoxyethane. It is a halogenated ether in form of a clear, colorless liquid, and its vapor has a strong fruity aroma. It is miscible with ethanol, acetone, chloroform, diethyl ether, and fixed oils. It is soluble in rubber.[10]
With a minimum alveolar concentration (MAC) of 0.16%,[53] methoxyflurane is an extremely potent anesthetic agent. It is a powerful analgesic agent at well below full anesthetic concentrations.[16][54][55][56][36] Because of its low volatility and very high boiling point (104.8 °C at 1 atmosphere), methoxyflurane has a low vapor pressure at ambient temperature and atmospheric pressure. It is therefore quite difficult to vaporize methoxyflurane using conventional anesthetic vaporizers.
| Property | Value[10][28][57] |
|---|---|
| Boiling point (at 1 atmosphere) | 104.8 °C |
| Minimum alveolar concentration (MAC) | 0.16%[53] |
| Vapor pressure (mmHg at 20 °C) | 22.5 |
| Partition coefficient (Blood:Gas) | 12 |
| Partition coefficient (Oil:Gas) | 950 |
| Partition coefficient (Oil:Water) | 400 |
| Specific gravity at 25 °C | 1.42 |
| Flash point | 63 °C |
| Molecular weight (g mol−1) | 164.97 |
| Vapor-liquid equilibrium (mL) | 208 |
| Flammability limits | 7% in air |
| Chemical stabilizer necessary | Yes |
The carbon–fluorine bond, a component of all organofluorine compounds, is the strongest chemical bond in organic chemistry.[58] Furthermore, this bond becomes shorter and stronger as more fluorine atoms are added to the same carbon on a given molecule. Because of this, fluoroalkanes are some of the most chemically stable organic compounds.
History
Methoxyflurane has been used since the 1970s in Australia as an emergency analgesic for short-term use, mostly by the Australian and New Zealand Defence Forces,[15] the Australian ambulance services,[12][37][38] and since 2018 by some Emergency medical services in Germany.[59]
All of the currently used volatile anesthetic agents are organofluorine compounds. Aside from the synthesis of Freon (Thomas Midgley, Jr. and Charles F. Kettering, 1928)[60] and the discovery of Teflon (Roy J. Plunkett, 1938),[61] the field of organofluorine chemistry had not attracted a great deal of attention up to 1940 because of the extreme reactivity of elemental fluorine, which had to be produced in situ for use in chemical reactions. The development of organofluorine chemistry was a spin-off from the Manhattan Project, during which elemental fluorine was produced on an industrial scale for the first time.
The need for fluorine arose from the need to separate the isotope 235U from 238U because the former, present in natural uranium at a concentration of less than 1% is fissile (capable of sustaining a nuclear chain reaction of nuclear fission with thermal neutrons),[62] whereas the latter is not. Members of the MAUD Committee (especially Francis Simon and Nicholas Kurti) proposed the use of gaseous diffusion for isotope separation, since, according to Graham's law the rate of diffusion is inversely proportional to molecular mass.[63] After an extensive search, uranium hexafluoride, UF6, was determined to be the most suitable compound of uranium to be used for the gaseous diffusion process.[64] Elemental fluorine is needed in the production of UF6.
Obstacles had to be overcome in the handling of both fluorine and UF6. Before the K-25 gaseous diffusion plant could be built, it was first necessary to develop non-reactive chemical compounds that could be used as coatings, lubricants and gaskets for the surfaces which would come into contact with the UF6 gas (a highly reactive and corrosive substance). William T. Miller,[65] professor of organic chemistry at Cornell University, was co-opted to develop such materials, because of his expertise in organofluorine chemistry. Miller and his team developed several novel non-reactive chlorofluorocarbon polymers that were used in this application.
Miller and his team continued to develop organofluorine chemistry after the end of World War II and methoxyflurane was made in 1948.[66]
In 1968, Robert Wexler of Abbott Laboratories developed the Analgizer, a disposable inhaler that allowed the self-administration of methoxyflurane vapor in air for analgesia.[67] The Analgizer consisted of a polyethylene cylinder 5 inches long and 1 inch in diameter with a 1 inch long mouthpiece. The device contained a rolled wick of polypropylene felt which held 15 milliliters of methoxyflurane. Because of the simplicity of the Analgizer and the pharmacological characteristics of methoxyflurane, it was easy for patients to self-administer the drug and rapidly achieve a level of conscious analgesia which could be maintained and adjusted as necessary over a period of time lasting from a few minutes to several hours. The 15 milliliter supply of methoxyflurane would typically last for two to three hours, during which time the user would often be partly amnesic to the sense of pain; the device could be refilled if necessary.[42] The Analgizer was found to be safe, effective, and simple to administer in obstetric patients during childbirth, as well as for patients with bone fractures and joint dislocations,[42] and for dressing changes on burn patients.[41] When used for labor analgesia, the Analgizer allows labor to progress normally and with no apparent adverse effect on Apgar scores.[42] All vital signs remain normal in obstetric patients, newborns, and injured patients.[42] The Analgizer was widely utilized for analgesia and sedation until the early 1970s, in a manner that foreshadowed the patient-controlled analgesia infusion pumps of today.[39][40][43][44] The Analgizer inhaler was withdrawn in 1974, but use of methoxyflurane as a sedative and analgesic continues in Australia and New Zealand in the form of the Penthrox inhaler.[15][12][16][13] Trials of methoxyflurane as an analgesic in emergency medicine are going on in the UK.
Notes
- 1 2 3 4 5 6 7 8 9 10 11 12 13 National Prescribing Service (2010). "Methoxyflurane (Penthrox) for analgesia (doctor's bag listing)". NPS RADAR. Canberra, Australia: National Prescribing Service, Department of Health and Ageing. Archived from the original on 2011-07-27. Retrieved 2011-06-12.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 6 August 2020. Retrieved 9 September 2020.
- 1 2 3 4 5 6 7 8 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. X. ISBN 9780857113382.
- 1 2 Jephcott, C; Grummet, J; Nguyen, N; Spruyt, O (May 2018). "A review of the safety and efficacy of inhaled methoxyflurane as an analgesic for outpatient procedures". British Journal of Anaesthesia. 120 (5): 1040–1048. doi:10.1016/j.bja.2018.01.011. PMID 29661381.
- ↑ Hardman, Jonathan G.; Hopkins, Philip M.; Struys, Michel M. R. F. (2017). Oxford Textbook of Anaesthesia. Oxford University Press. p. 553. ISBN 9780199642045. Archived from the original on 2019-01-07. Retrieved 2019-01-07.
- 1 2 Mazze Richard I (2006). "Methoxyflurane Revisited: Tale of an Anesthetic from Cradle to Grave". Anesthesiology. 105 (4): 843–846. doi:10.1097/00000542-200610000-00031. PMID 17006084.
- ↑ "NZ Medsafe Datasheet" (PDF). Archived (PDF) from the original on 2019-12-31.
- ↑ PHECC Clinical Practice Guidelines (6 ed.). Pre-Hospital Emergency Care Council. March 2017. p. 100. ISBN 978-0-9929363-6-5. Archived from the original on 2018-08-15. Retrieved 7 January 2019.
- ↑ Porter, KM; Siddiqui, MK; Sharma, I; Dickerson, S; Eberhardt, A (2018). "Management of trauma pain in the emergency setting: low-dose methoxyflurane or nitrous oxide? A systematic review and indirect treatment comparison". Journal of Pain Research. 11: 11–21. doi:10.2147/JPR.S150600. PMC 5741984. PMID 29302193.
- 1 2 3 Medical Developments International Pty. Ltd. (2009). "Penthrox (methoxyflurane) inhalation: product information" (PDF). Springvale, Victoria, Australia: Medical Developments International Limited. Archived (PDF) from the original on 2012-03-15. Retrieved 2011-06-12.
- ↑ Wellness on Wellington (2010). "The green whistle" (PDF). Wellnews. 12 (2): 1. Archived from the original (PDF) on 2011-03-29.
- 1 2 3 4 5 6 Babl FE, Jamison SR, Spicer M, Bernard S (2006). "Inhaled methoxyflurane as a prehospital analgesic in children". Emergency Medicine Australasia. 18 (4): 404–10. doi:10.1111/j.1742-6723.2006.00874.x. PMID 16842312.
- 1 2 3 4 Grindlay J, Babl FE (2009). "Efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital setting". Emergency Medicine Australasia. 21 (1): 4–11. doi:10.1111/j.1742-6723.2009.01153.x. PMID 19254307.
- ↑ Rossi, S, ed. (2009). Australian Medicines Handbook (10th ed.). Adelaide: Australian Medicines Handbook Pty, Ltd. ISBN 978-0-9757919-9-8.
- 1 2 3 4 McLennan JV (2007). "Is methoxyflurane a suitable battlefield analgesic?" (PDF). Journal of the Royal Army Medical Corps. 153 (2): 111–3. doi:10.1136/jramc-153-02-08. PMID 17896540. Archived from the original (PDF) on 2011-07-15.
- 1 2 3 4 Babl F, Barnett P, Palmer G, Oakley E, Davidson A (2007). "A pilot study of inhaled methoxyflurane for procedural analgesia in children". Pediatric Anesthesia. 17 (2): 148–53. doi:10.1111/j.1460-9592.2006.02037.x. PMID 17238886.
- 1 2 Cousins MJ, Mazze RI (1973). "Methoxyflurane nephrotoxicity: a study of dose response in man (abstract)". Journal of the American Medical Association. 225 (13): 1611–6. doi:10.1001/jama.1973.03220410023005. PMID 4740737.
- 1 2 Gottlieb LS, Trey C (1974). "The effects of fluorinated anesthetics on the liver and kidneys". Annual Review of Medicine. 25: 411–29. doi:10.1146/annurev.me.25.020174.002211. PMID 4596236.
- ↑ "CDC - NIOSH Pocket Guide to Chemical Hazards - Methoxyflurane". www.cdc.gov. Archived from the original on 2015-11-20. Retrieved 2015-11-19.
- ↑ Paddock, RB, Parker JW, Guadagni NP (1964). "The effects of methoxyflurane on renal function". Anesthesiology. 25: 707–8. PMID 14211499.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 Crandell WB, Pappas SG, Macdonald A (1966). "Nephrotoxicity associated with methoxyflurane anesthesia". Anesthesiology. 27 (5): 591–607. doi:10.1097/00000542-196609000-00010. PMID 5918999.
- ↑ Barash, Cullen and Stoelting (2009), Ebert and Schmid, Chapter 17: Inhaled Anesthetics, pp. 413–43
- ↑ Helsinn Birex Therapeutics Ltd (2009). "By-Mycin 50mg capsules". medicines.ie: Medicines information online. Dublin, Ireland: Irish Pharmaceutical Healthcare Association Ltd. Archived from the original on 2011-07-21. Retrieved 2011-06-12.
- ↑ Jones NO (1972). "Methoxyflurane nephrotoxicity – a review and a case report". Canadian Anaesthetists' Society Journal. 19 (2): 152–9. doi:10.1007/BF03005045. PMID 5029469.
- ↑ Mazze RI (1976). "Methoxyflurane nephropathy". Environmental Health Perspectives. 15: 111–9. doi:10.1289/ehp.7615111. JSTOR 3428393. PMC 1475154. PMID 1001288.
- ↑ Kharasch ED, Schroeder JL, Liggitt HD, Park SB, Whittington D, Sheffels P (2006). "New insights into the mechanism of methoxyflurane nephrotoxicity and implications for anesthetic development (part 1): Identification of the nephrotoxic metabolic pathway". Anesthesiology. 105 (4): 726–36. doi:10.1097/00000542-200610000-00019. PMID 17006072.
- ↑ Kharasch ED, Schroeder JL, Liggitt HD, Ensign D, Whittington D (2006). "New insights into the mechanism of methoxyflurane nephrotoxicity and implications for anesthetic development (part 2): Identification of nephrotoxic metabolites". Anesthesiology. 105 (4): 737–45. doi:10.1097/00000542-200610000-00020. PMID 17006073.
- 1 2 3 4 5 Wyant GM, Chang CA, Rapicavoli E (1961). "Methoxyflurane (penthrane): a laboratory and clinical study". Canadian Anaesthetists' Society Journal. 8 (5): 477–87. doi:10.1007/BF03021373. PMID 13786945.
- 1 2 3 Siebecker KL, James M, Bamforth BJ, Orth OS (1961). "The respiratory effect of methoxyflurane on dog and man". Anesthesiology. 22 (1): 143. doi:10.1097/00000542-196101000-00044.
- ↑ Millar RA, Morris ME (1961). "A study of methoxyflurane anaesthesia". Canadian Anaesthetists' Society Journal. 8 (3): 210–5. doi:10.1007/BF03028110. PMID 13770698.
- ↑ Artusio JF, Van Poznak A, Hunt RE, Tiers RM, Alexander MA (1960). "A clinical evaluation of methoxyflurane in man". Anesthesiology. 21 (5): 512–7. doi:10.1097/00000542-196009000-00009. PMID 13794589.
- ↑ Van Poznak A, Artusio JF (1960). "Anesthetic properties of a series of fluorinated compounds: I. fluorinated hydrocarbons". Toxicology and Applied Pharmacology. 2 (4): 363–73. doi:10.1016/0041-008X(60)90002-8. PMID 13841124.
- ↑ Van Poznak A, Artusio JF (1960). "Anesthetic properties of a series of fluorinated compounds: II. fluorinated ethers". Toxicology and Applied Pharmacology. 2 (4): 374–8. doi:10.1016/0041-008X(60)90003-X. PMID 13841125.
- ↑ Boisvert M, Hudon F (1962). "Clinical evaluation of methoxyflurane in obstetrical anaesthesia: A report on 500 cases". Canadian Anaesthetists' Society Journal. 9 (4): 325–30. doi:10.1007/BF03021269. PMID 13870700.
- ↑ Romagnoli A, Korman D (1962). "Methoxyflurane in obstetrical anaesthesia and analgesia". Canadian Anaesthetists' Society Journal. 9 (5): 414–8. doi:10.1007/BF03019135. PMID 14493514.
- 1 2 Crankshaw DP (2005). "Methoxyflurane for relief of acute pain: interpretation of uptake and elimination curves (abstract)". Anesthesiology. 103 (Supplement): A756. Archived from the original on 2013-01-17.
- 1 2 Buntine P, Thom O, Babl F, Bailey M, Bernard S (2007). "Prehospital analgesia in adults using inhaled methoxyflurane". Emergency Medicine Australasia. 19 (6): 509–14. doi:10.1111/j.1742-6723.2007.01017.x. PMID 18021102.
- 1 2 Johnston S, Wilkes GJ, Thompson JA, Ziman M, Brightwell R (2011). "Inhaled methoxyflurane and intranasal fentanyl for prehospital management of visceral pain in an Australian ambulance service". Emergency Medicine Journal. 28 (1): 57–63. doi:10.1136/emj.2009.078717. PMID 20466829. Archived from the original on 2019-04-26. Retrieved 2019-01-27.
- 1 2 3 Major V, Rosen M, Mushin WW (1966). "Methoxyflurane as an obstetric analgesic: a comparison with trichloroethylene". British Medical Journal. 2 (5529): 1554–61. doi:10.1136/bmj.2.5529.1554. PMC 1944957. PMID 5926260.
- 1 2 Dragon A, Goldstein I (1967). "Methoxyflurane: preliminary report on analgesic and mood modifying properties in dentistry". Journal of the American Dental Association. 75 (5): 1176–81. doi:10.14219/jada.archive.1967.0358. PMID 5233333.
- 1 2 Packer KJ, Titel JH (1969). "Methoxyflurane analgesia for burns dressings: experience with the Analgizer". British Journal of Anaesthesia. 41 (12): 1080–5. CiteSeerX 10.1.1.1028.6601. doi:10.1093/bja/41.12.1080. PMID 4903969.
- 1 2 3 4 5 Romagnoli A, Busque L, Power DJ (1970). "The "analgizer" in a general hospital: a preliminary report". Canadian Anaesthetists' Society Journal. 17 (3): 275–8. doi:10.1007/BF03004607. PMID 5512851.
- 1 2 Firn S (1972). "Methoxyflurane analgesia for burns dressings and other painful ward procedures in children". British Journal of Anaesthesia. 44 (5): 517–22. doi:10.1093/bja/44.5.517. PMID 5044082. Archived from the original on 2021-09-23. Retrieved 2019-12-23.
- 1 2 Josephson CA, Schwartz W (1974). "The Cardiff inhaler and Penthrane. A method of sedation analgesia in routine dentistry". Journal of the Dental Association of South Africa. 29 (2): 77–80. PMID 4534883.
- ↑ Lewis LA (1984). "Methoxyflurane analgesia for office surgery: surgical gem". Journal of Dermatologic Surgery and Oncology. 10 (2): 85–6. doi:10.1111/j.1524-4725.1984.tb01191.x. PMID 6693612.
- ↑ Komesaroff D (1995). "Pre-hospital pain relief: Penthrane or Entonox". Australian Journal of Emergency Care. 2 (2): 28–9. ISSN 1322-3127.
- ↑ Chin R, McCaskill M, Browne G, Lam L (2002). "A randomised controlled trial of inhaled methoxyflurane pain relief in children with upper limb fracture (abstract)". Journal of Paediatrics and Child Health. 38 (5): A13–4. doi:10.1046/j.1440-1754.2002.00385.x. ISSN 1034-4810.
- ↑ Nicholas P. Franks (2006). "Molecular targets underlying general anaesthesia". British Journal of Pharmacology. 147 Suppl 1: S72–S81. doi:10.1038/sj.bjp.0706441. PMC 1760740. PMID 16402123.
- ↑ P.-L. Chau (2010). "New insights into the molecular mechanisms of general anaesthetics". British Journal of Pharmacology. 161 (2): 288–307. doi:10.1111/j.1476-5381.2010.00891.x. PMC 2989583. PMID 20735416.
- ↑ A. Jenkins, N. P. Franks & W. R. Lieb (1999). "Effects of temperature and volatile anesthetics on GABA(A) receptors". Anesthesiology. 90 (2): 484–491. doi:10.1097/00000542-199902000-00024. PMID 9952156.
- ↑ M. D. Krasowski & N. L. Harrison (2000). "The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations". British Journal of Pharmacology. 129 (4): 731–743. doi:10.1038/sj.bjp.0703087. PMC 1571881. PMID 10683198.
- ↑ S. J. Mihic, Q. Ye, M. J. Wick, V. V. Koltchine, M. D. Krasowski, S. E. Finn, M. P. Mascia, C. F. Valenzuela, K. K. Hanson, E. P. Greenblatt, R. A. Harris & N. L. Harrison (1997). "Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors". Nature. 389 (6649): 385–389. Bibcode:1997Natur.389..385M. doi:10.1038/38738. PMID 9311780.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 Mazze RI, Shue GL, Jackson SH (1971). "Renal Dysfunction Associated With Methoxyflurane Anesthesia". Journal of the American Medical Association. 216 (2): 278–288. doi:10.1001/jama.1971.03180280032006.
- ↑ Torda TAG (1963). "The analgesic effect of methoxyflurane". Anaesthesia. 18 (3): 287–9. doi:10.1111/j.1365-2044.1963.tb13548.x. PMID 13981361.
- ↑ Tomlin PJ, Jones BC, Edwards R, Robin PE (1973). "Subjective and objective sensory responses to inhalation of nitrous oxide and methoxyflurane". British Journal of Anaesthesia. 45 (7): 719–25. doi:10.1093/bja/45.7.719. PMID 4730164. Archived from the original on 2021-09-23. Retrieved 2019-12-23.
- ↑ Tomi K, Mashimo T, Tashiro C, Yagi M, Pak M, Nishimura S, Nishimura M, Yoshiya I (1993). "Alterations in pain threshold and psychomotor response associated with subanaesthetic concentrations of inhalational anaesthetics in humans". British Journal of Anaesthesia. 70 (6): 684–6. doi:10.1093/bja/70.6.684. PMID 8329263.
- ↑ McIntyre JW, Gain EA (1962). "Methoxyflurane". Canadian Anaesthetists' Society Journal. 9 (4): 319–24. doi:10.1007/BF03021268.
- ↑ O'Hagan D (2008). "Understanding organofluorine chemistry. An introduction to the C–F bond". Chemical Society Reviews. 37 (2): 308–19. doi:10.1039/b711844a. PMID 18197347.
- ↑ Blaschke2018
- ↑ Sneader (2005), Sneader W, Chapter 8: Systematic medicine, pp. 74–87
- ↑ DuPont (2010). "Roy Plunkett: 1938". DuPont Heritage. Wilmington, Delaware: E. I. du Pont de Nemours and Company. Archived from the original on 2012-02-17. Retrieved 2011-06-12.
- ↑ Cotton (2006), Cotton S, Chapter 10: Binary compounds of the actinides, pp. 155–72
- ↑ Rhodes (1986), Rhodes R, Chapter 11: Cross sections, pp. 318–56
- ↑ Beaton L (1962). "The slow-down in nuclear explosive production". New Scientist. 16 (309): 141–3.
{{cite journal}}: CS1 maint: url-status (link) - ↑ Friedlander Jr., BP (3 December 1998). "William T. Miller, Manhattan Project scientist and Cornell professor of chemistry, dies at 87". Cornell News. Ithaca, New York: Cornell University. Archived from the original on 7 June 2011. Retrieved 2011-06-12.
- ↑ Miller Jr, WT; Fager, EW; Griswold, PH (1948). "The Addition of Methyl Alcohol to Fluoroethylenes". Journal of the American Chemical Society. 70 (1): 431–2. doi:10.1021/ja01181a526.
- ↑ Wexler RE (1968). "Analgizer: Inhaler for supervised self-administration of inhalation anesthesia". Abbott Park, Illinois: Abbott Laboratories. Archived from the original on 2018-12-03. Retrieved 2011-06-12.
References
- Barash, PG; Cullen, BF; Stoelting, RK; Cahalan, MK; Stock, MC, eds. (2009). Clinical anesthesia (6th ed.). Philadelphia: Lippincott Williams & Wilkins. ISBN 978-0-7817-8763-5. Archived from the original on 2017-02-20. Retrieved 2016-09-24.
- Cotton S (2006). Lanthanide and actinide chemistry (1st ed.). Chichester, England: John Wiley and Sons, Ltd. ISBN 978-0-470-01006-8. Archived from the original on 2021-11-10. Retrieved 2022-03-14.
- Rhodes, R (1986). The making of the atomic bomb. New York: Simon & Schuster. ISBN 978-0-684-81378-3. Archived from the original on 2017-02-23. Retrieved 2016-09-24.
- Sneader W (2005). Drug discovery: a history. Chichester, England: John Wiley and Sons, Ltd. ISBN 978-0-471-89980-8.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- NIOSH Pocket Guide to Chemical Hazards Archived 2015-11-20 at the Wayback Machine