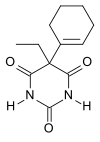
Cyclobarbital
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.127 |
| Chemical and physical data | |
| Formula | C12H16N2O3 |
| Molar mass | 236.271 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Cyclobarbital, also known as cyclobarbitol or cyclobarbitone, is a drug that is a barbiturate derivative.[1] It was available in Russia as a fixed-dose combination with diazepam (100 mg cyclobarbital + 10 mg diazepam; brand name Reladorm) for the treatment of insomnia but was discontinued in 2019.
References
- ↑ Breimer DD, Winten MA (March 1976). "Pharmacokinetics and relative bioavailability of cyclobarbital calcium in man after oral administration". European Journal of Clinical Pharmacology. 09 (5–6): 443–50. doi:10.1007/bf00606563. PMID 989475. S2CID 20271169.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.