Flutazolam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Coreminal (JP) |
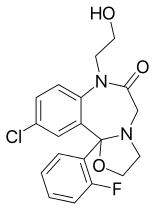
| Other names | 13-chloro- 2-(2-fluorophenyl)- 9-(2-hydroxyethyl)- 3-oxa- 6,9-diazatricyclo[8.4.0.02,6] tetradeca-1(10),11,13- trien- 8-one |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 3.5 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H18ClFN2O3 |
| Molar mass | 376.81 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Flutazolam[1] (Coreminal, MS-4101) is a drug which is a benzodiazepine derivative. It was invented in Japan, and this is the main country in which it has been used medically. It has sedative, muscle relaxant, anticonvulsant, and anxiolytic effects similar to those produced by other benzodiazepine derivatives, and though it is around the same potency as diazepam, it produces a more marked sedation and impaired coordination. It is indicated for the treatment of insomnia.[2] Its major active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam (trade name Dalmane).[3]
Flutazolam is closely related in structure to another benzodiazepine, haloxazolam.[4][5]
See also
References
- ↑ DE 1952486
- ↑ Mitsushima T, Ueki S. Psychopharmacological effects of flutazolam (MS-4101). Nippon Yakurigaku Zasshi. 1978 Nov;74(8):959-79. (Japanese).
- ↑ Miyaguchi H, Kuwayama K, Tsujikawa K, Kanamori T, Iwata YT, Inoue H, Kishi T (February 2006). "A method for screening for various sedative-hypnotics in serum by liquid chromatography/single quadrupole mass spectrometry". Forensic Science International. 157 (1): 57–70. doi:10.1016/j.forsciint.2005.03.011. PMID 15869852.
- ↑ Kuwayama T, Kurono Y, Muramatsu T, Yashiro T, Ikeda K (January 1986). "The behavior of 1,4-benzodiazepine drugs in acidic media. V. Kinetics of hydrolysis of flutazolam and haloxazolam in aqueous solution". Chemical and Pharmaceutical Bulletin (Tokyo). 34 (1): 320–6. doi:10.1248/cpb.34.320. PMID 2870816.
- ↑ Yashiro T, Kuwayama T, Kawazura H, Suzuki T (October 1987). "[The behavior of 1,4-benzodiazepine drugs in acidic media. IX. Effect of hydrolyzate of flutazolam on the central nervous system]". Yakugaku Zasshi : Journal of the Pharmaceutical Society of Japan (in Japanese). 107 (10): 830–4. doi:10.1248/yakushi1947.107.10_830. PMID 2894449.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.