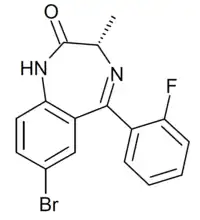
SH-I-048A
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C16H12BrFN2O |
| Molar mass | 347.187 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
SH-I-048A (SH-i-048A) is a benzodiazepine derivative related in structure to compounds such as flubromazepam and meclonazepam. SH-I-048A is described as a non subtype selective superagonist at the benzodiazepine site of GABAA receptors,[1] with a binding affinity of 0.77nM at the α1 subtype, 0.17nM at α2, 0.38nM at α3 and 0.11nM at α5.[2] It has been used to study the functional differences between the different subtypes of the GABAA receptor.[3][4]
See also
References
- ↑ Obradović AL, Joksimović S, Poe MM, Ramerstorfer J, Varagic Z, Namjoshi O, Batinić B, Radulović T, Marković B, Roth BL, Sieghart W, Cook JM, Savić MM. "Sh-I-048A, an in vitro non-selective super-agonist at the benzodiazepine site of GABAA receptors: the approximated activation of receptor subtypes may explain behavioral effects". Brain Research. 1554.
- ↑ Clayton T, Poe MM, Rallapalli S, Biawat P, Savić MM, Rowlett JK, et al. (2015). "A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model". International Journal of Medicinal Chemistry. 2015: 430248. doi:10.1155/2015/430248. PMC 4657098. PMID 26682068.
- ↑ Obradović LA, Joksimović S, Poe MM, Timić T, Cook JM, Savić MM (June 2014). "Delayed Behavioral Effects of SH–I–048A, a Novel Nonselective Positive Modulator of Gabaa Receptors, After Peripheral Nerve Injury in Rats". Acta Veterinaria. 64 (2): 189–99. doi:10.2478/acve-2014-0018.
- ↑ Elgarf AA, Siebert DC, Steudle F, Draxler A, Li G, Huang S, et al. (August 2018). "Different Benzodiazepines Bind With Distinct Binding Modes to GABAA Receptors". ACS Chemical Biology. 13 (8): 2033–2039. doi:10.1021/acschembio.8b00144. PMC 6102643. PMID 29767950.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.