Talampanel
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.001 |
| Chemical and physical data | |
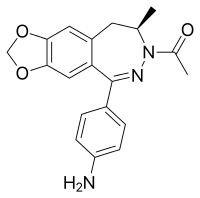
| Formula | C19H19N3O3 |
| Molar mass | 337.379 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Talampanel (INN; development codes GYKI 537773 and LY300164) is a drug which has been investigated for the treatment of epilepsy,[1][2] malignant gliomas,[3] and amyotrophic lateral sclerosis (ALS).[4]
As of May 2010, results from the trial for ALS have been found negative.[5] Talampanel is not currently under development.
Talampanel acts as a non-competitive antagonist of the AMPA receptor, a type of ionotropic glutamate receptor in the central nervous system.[6]
It showed effectiveness for epilepsy in clinical trials but its development was suspended due to its poor pharmacokinetic profile, namely a short terminal half-life (3 hours) that necessitated multiple doses per day.[7]
References
- ↑ Luszczki, J. J. (2009). "Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions". Pharmacological Reports. 61 (2): 197–216. doi:10.1016/s1734-1140(09)70024-6. PMID 19443931.
- ↑ Bialer, M.; Johannessen, S. I.; Kupferberg, H. J.; Levy, R. H.; Perucca, E.; Tomson, T. R. (2007). "Progress report on new antiepileptic drugs: A summary of the Eighth Eilat Conference (EILAT VIII)". Epilepsy Research. 73 (1): 1–52. doi:10.1016/j.eplepsyres.2006.10.008. PMID 17158031. S2CID 45026113.
- ↑ Iwamoto, F. M.; Kreisl, T. N.; Kim, L.; Duic, J. P.; Butman, J. A.; Albert, P. S.; Fine, H. A. (2010). "Phase II Trial of Talampanel, a Glutamate Receptor Inhibitor, for Adults with Recurrent Malignant Gliomas". Cancer. 116 (7): 1776–1782. doi:10.1002/cncr.24957. PMC 2846997. PMID 20143438.
- ↑ Pascuzzi, R. M.; Shefner, J.; Chappell, A. S.; Bjerke, J. S.; Tamura, R.; Chaudhry, V.; Clawson, L.; Haas, L.; Rothstein, J. D. (2009). "A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis". Amyotrophic Lateral Sclerosis. 11 (3): 266–271. doi:10.3109/17482960903307805. PMID 19961264. S2CID 7388452.
- ↑ Talampanel Trial, Talampanel Trial on alsa.org
- ↑ Aujla, P. K.; Fetell, M. R.; Jensen, F. E. (2009). "Talampanel suppresses the acute and chronic effects of seizures in a rodent neonatal seizure model". Epilepsia. 50 (4): 694–701. doi:10.1111/j.1528-1167.2008.01947.x. PMC 2672962. PMID 19220413.
- ↑ Lee K, Goodman L, Fourie C, Schenk S, Leitch B, Montgomery JM (2016). "AMPA Receptors as Therapeutic Targets for Neurological Disorders". Adv Protein Chem Struct Biol. Advances in Protein Chemistry and Structural Biology. 103: 203–61. doi:10.1016/bs.apcsb.2015.10.004. ISBN 9780128047941. PMID 26920691.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.