Psychotridine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
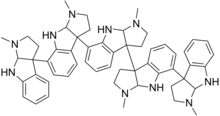
| Formula | C55H62N10 |
| Molar mass | 863.171 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Psychotridine is an alkaloid found in some species of the genus Psychotria, namely Psychotria colorata,[1] but also Psychotria forsteriana,[2] Psychotria lyciiflora,[3] Psychotria oleoides,[3] and Psychotria beccarioides.[4] Psychotridine has analgesic effects and dose-dependently inhibits dizocilpine binding to cortical membranes in vitro, suggesting that it acts as a non-competitive NMDA receptor antagonist.[1]
See also
References
- 1 2 Amador TA, Verotta L, Nunes DS, Elisabetsky E (May 2001). "Involvement of NMDA receptors in the analgesic properties of psychotridine". Phytomedicine. 8 (3): 202–6. doi:10.1078/0944-7113-00025. PMID 11417913.
- ↑ Beretz A, Roth-Georger A, Corre G, Kuballa B, Anton R, Cazenave JP (August 1985). "Polyindolinic Alkaloids from Psychotria forsteriana. Potent Inhibitors of the Aggregation of Human Platelets". Planta Medica. 51 (4): 300–3. doi:10.1055/s-2007-969496. PMID 17340518.
- 1 2 Jannic V, Guéritte F, Laprévote O, et al. (June 1999). "Pyrrolidinoindoline alkaloids from Psychotria oleoides and Psychotria lyciiflora". Journal of Natural Products. 62 (6): 838–43. doi:10.1021/np9805387. PMID 10395499.
- ↑ Hart, N.; Johns, S.; Lamberton, J.; Summons, R. (1974). "Psychotridine, a C55H62N10 alkaloid from Psychotria beccarioides (Rubiaceae)". Australian Journal of Chemistry. 27 (3): 639–646. doi:10.1071/ch9740639.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.