Dasolampanel
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
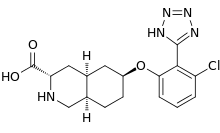
| Formula | C17H20ClN5O3 |
| Molar mass | 377.83 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dasolampanel (INN, USAN, code name NGX-426) is an orally bioavailable analog of tezampanel and thereby competitive antagonist of the AMPA and kainate receptors which was under development by Raptor Pharmaceuticals/Torrey Pines Therapeutics for the treatment of chronic pain conditions including neuropathic pain and migraine.[1] It was developed as a follow-on compound to tezampanel, as tezampanel is not bioavailable orally and must be administered by intravenous injection,[2][3] but ultimately neither drug was ever marketed.
See also
References
- ↑ Stolerman IP (31 July 2010). Encyclopedia of Psychopharmacology. Springer Science & Business Media. pp. 514–. ISBN 978-3-540-68698-9.
- ↑ Olesen J, Ramadan N (21 August 2008). Innovative Drug Development for Headache Disorders. Oxford University Press. pp. 188–. ISBN 978-0-19-955276-4.
- ↑ Firestein GS, Budd R, Gabriel SE, O'Dell JR, McInnes IB (31 August 2012). Kelley's Textbook of Rheumatology: Expert Consult Premium Edition: Enhanced Online Features. Elsevier Health Sciences. pp. 1031–. ISBN 978-1-4557-3767-3.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.