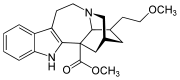
18-Methoxycoronaridine
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H28N2O3 |
| Molar mass | 368.477 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
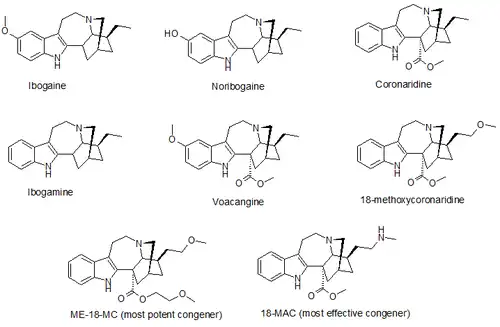
18-Methoxycoronaridine (18-MC) is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose.[1][2] It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.[3]
18-MC was in the early stages of human testing by the California-based drug development company Savant HWP before being acquired by MindMed, a Canadian pharmaceutical company newly listed on the NASDAQ in April 2021.[4] [5] In 2002 the research team began raising funds for human trials, but were unable to secure the estimated $5 million needed.[6] In 2010, Obiter Research, a chemical manufacturer in Champaign, Illinois, signed a patent license with Albany Medical College and the University of Vermont, allowing them the right to synthesize and market 18-MC and other congeners. In 2012 the National Institute on Drug Abuse gave a $6.5 million grant to Savant HWP for human trials.[5] In 2017 it went into Phase-II trials in Brazil for treatment of Leishmaniasis at the Evandro Chagas Institute,[7] but not for approval for use as a treatment for drug addiction.
Pharmacology
18-MC is a α3β4 nicotinic antagonist and, in contrast to ibogaine, has no affinity at the α4β2 subtype nor at NMDA-channels nor at the serotonin transporter,[8] and has significantly reduced affinity for sodium channels and for the σ receptor, but retains modest affinity for μ-opioid receptors where it acts as an agonist,[9] and κ-opioid receptors.[10] The sites of action in the brain include the medial habenula, interpeduncular nucleus,[11][12][13] dorsolateral tegmentum and basolateral amygdala.[14] (±)-18-MC competitively inhibits α9α10 nAChRs with potencies higher than that at α3β4 and α4β2 nAChRs and directly blocks CaV2.2.[15]
Chemistry
Derivatives
A number of derivatives of 18-MC have been developed, with several of them being superior to 18-MC itself, the methoxyethyl congener ME-18-MC being more potent than 18-MC with similar efficacy, and the methylamino analogue 18-MAC being more effective than 18-MC with around the same potency. These compounds were also found to act as selective α3β4 nicotinic acetylcholine antagonists, with little or no effect on NMDA receptors.[16][17]
See also
- Coronaridine
- Ibogaine
- Noribogaine
- Voacangine
References
- ↑ Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH (May 1996). "18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats". Brain Research. 719 (1–2): 29–35. doi:10.1016/0006-8993(96)00056-X. PMID 8782860. S2CID 6178161.
- ↑ Glick SD, Sell EM, Maisonneuve IM (December 2008). "Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration". European Journal of Pharmacology. 599 (1–3): 91–5. doi:10.1016/j.ejphar.2008.09.038. PMC 2600595. PMID 18930043.
- ↑ Taraschenko OD, Rubbinaccio HY, Maisonneuve IM, Glick SD (December 2008). "18-methoxycoronaridine: a potential new treatment for obesity in rats?". Psychopharmacology. 201 (3): 339–50. doi:10.1007/s00213-008-1290-9. PMC 3787601. PMID 18751969.
- ↑ Mindmed Acquires Opioid Addiction Drug Candidate Based on the Natural Psychedelic Ibogaine newswire.ca September 16, 2019.
- 1 2 Albany Med scientist closer to addiction drug success timesunion.com June 27, 2014.
- ↑ Addiction Treatment Strives for Legitimacy. Journal of the American Medical Association. 2002; 288: 3096-3101.
- ↑ "Phase 2 Trial to Evaluate 18-Methoxycoronaridine Efficacy, Safety and Tolerability in Cutaneous Leishmaniasis Patients". ClinicalTrials.gov. Retrieved 19 February 2020.
- ↑ Maisonneuve IM, Glick SD (June 2003). "Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment". Pharmacology, Biochemistry, and Behavior. 75 (3): 607–18. doi:10.1016/S0091-3057(03)00119-9. PMID 12895678. S2CID 26758480.
- ↑ Antonio T, Childers SR, Rothman RB, Dersch CM, King C, Kuehne M, et al. (2013). "Effect of Iboga alkaloids on µ-opioid receptor-coupled G protein activation". PLOS ONE. 8 (10): e77262. Bibcode:2013PLoSO...877262A. doi:10.1371/journal.pone.0077262. PMC 3818563. PMID 24204784.
- ↑ Glick SD, Maisonneuve IM, Hough LB, Kuehne ME, Bandarage UK. (±)-18-Methoxycoronaridine: A Novel Iboga Alkaloid Congener Having Potential Anti-Addictive Efficacy. CNS Drug Reviews 1999;5(1):27-42.
- ↑ Glick SD, Ramirez RL, Livi JM, Maisonneuve IM (May 2006). "18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats". European Journal of Pharmacology. 537 (1–3): 94–8. doi:10.1016/j.ejphar.2006.03.045. PMID 16626688.
- ↑ Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD (July 2007). "18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens". Synapse. 61 (7): 547–60. doi:10.1002/syn.20396. PMID 17447255. S2CID 2252348.
- ↑ Taraschenko OD, Rubbinaccio HY, Shulan JM, Glick SD, Maisonneuve IM (July 2007). "Morphine-induced changes in acetylcholine release in the interpeduncular nucleus and relationship to changes in motor behavior in rats". Neuropharmacology. 53 (1): 18–26. doi:10.1016/j.neuropharm.2007.04.010. PMC 2025684. PMID 17544456.
- ↑ Glick SD, Sell EM, Maisonneuve IM (December 2008). "Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration". European Journal of Pharmacology. 599 (1–3): 91–5. doi:10.1016/j.ejphar.2008.09.038. PMC 2600595. PMID 18930043.
- ↑ Arias HR, Tae HS, Micheli L, Yousuf A, Ghelardini C, Adams DJ, Di Cesare Mannelli L (September 2020). "Coronaridine congeners decrease neuropathic pain in mice and inhibit α9α10 nicotinic acetylcholine receptors and CaV2.2 channels". Neuropharmacology. 175: 108194. doi:10.1016/j.neuropharm.2020.108194. hdl:2158/1213504. PMID 32540451. S2CID 219705597.
- ↑ Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, et al. (June 2003). "Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents". Journal of Medicinal Chemistry. 46 (13): 2716–30. doi:10.1021/jm020562o. PMID 12801235.
- ↑ Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW (May 2004). "Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration". European Journal of Pharmacology. 492 (2–3): 159–67. doi:10.1016/j.ejphar.2004.03.062. PMID 15178360.