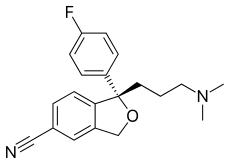
Citalopram
 (R)-(−)-citalopram (top), (S)-(+)-citalopram (bottom) | |
| Names | |
|---|---|
| Pronunciation | /saɪˈtæləˌpræm, sɪ-/;[1] |
| Trade names | Celexa, Cipramil, others (see below) |
IUPAC name
| |
| Clinical data | |
| Drug class | Selective serotonin reuptake inhibitor (SSRI)[2] |
| Main uses | Major depressive disorder, obsessive compulsive disorder, panic disorder, social phobia[2] |
| Side effects | Nausea, trouble sleeping, sexual problems, shakiness, feeling tired, sweating[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 20 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699001 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 80% peak at 4 h[2] |
| Metabolism | Liver (CYP3A4 and CYP2C19) |
| Metabolites | Desmethylcitalopram (DCT) and didesmethylcitalopram (DDCT) |
| Elimination half-life | 35 h |
| Excretion | Mostly as unmetabolized citalopram, partly DCT and traces of DDCT in urine |
| Chemical and physical data | |
| Formula | C20H21FN2O |
| Molar mass | 324.399 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[2] It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and social phobia.[2] The antidepressant effects may take one to four weeks to occur.[2] It is taken by mouth.[2]
Common side effects include nausea, trouble sleeping, sexual problems, shakiness, feeling tired, and sweating.[2] Serious side effects include an increased risk of suicide in those under the age of 25, serotonin syndrome, glaucoma, and QT prolongation.[2] It should not be used in someone on a MAO inhibitor.[2] Antidepressant discontinuation syndrome may occur when stopped.[2] There are concerns that use during pregnancy may harm the baby.[4]
Citalopram was approved for medical use in the United States in 1998.[2] It is on the World Health Organization's List of Essential Medicines as an alternative to fluoxetine.[5] It is available as a generic medication.[6] In the United Kingdom, a typical dose costs the NHS less than 20 GBP per month.[6] In the United States, it costs 50 to 100 USD per month as of 2016.[7] In 2017, it was the 26th most commonly prescribed medication in the United States, with more than 24 million prescriptions.[8][9]
Medical uses

Depression
In the National Institute for Health and Clinical Excellence ranking of ten antidepressants for efficacy and cost-effectiveness[10] citalopram is fifth in effectiveness (after mirtazapine, escitalopram, venlafaxine, and sertraline) and fourth in cost-effectiveness. The ranking results were based on the meta-analysis by Andrea Cipriani.[11] In another analysis by Cipriani, citalopram was found to be more efficacious than paroxetine and reboxetine, and more acceptable than tricyclics, reboxetine, and venlafaxine, but less efficacious than escitalopram.[12]
Evidence for effectiveness of citalopram for treating depression in children is uncertain.[13][14]
Panic disorder
Citalopram is licensed in the UK and other European countries[15] for panic disorder, with or without agoraphobia.
Other
Citalopram may be used off-label to treat anxiety, and dysthymia,[16] premenstrual dysphoric disorder, body dysmorphic disorder, and obsessive–compulsive disorder.[17]
It has been shown to be effective in 85% of patients with generalized anxiety disorder, including some who had failed with other SSRIs.[18] It also appears to be as effective as fluvoxamine and paroxetine in obsessive–compulsive disorder.[19] Some data suggest the effectiveness of intravenous infusion of citalopram in resistant OCD.[20] Citalopram is well tolerated and as effective as moclobemide in social anxiety disorder.[21] There are studies suggesting that citalopram can be useful in reducing aggressive and impulsive behavior.[22][23] It appears to be superior to placebo for behavioural disturbances associated with dementia.[24] It has also been used successfully for hypersexuality in early Alzheimer's disease.[25]
A meta-analysis, including studies with fluoxetine, paroxetine, sertraline, escitalopram, and citalopram versus placebo, showed SSRIs to be effective in reducing symptoms of premenstrual syndrome, whether taken continuously or just in the luteal phase.[26] Citalopram has produced a modest reduction in alcoholic drink intake and increase in drink-free days in studies of alcoholics, possibly by decreasing desire or reducing the reward.[27]
Citalopram has been found to reduce the symptoms of diabetic neuropathy.[28]
While on its own citalopram is less effective than amitriptyline in the prevention of migraines, in refractory cases, combination therapy may be more effective.[29]
Citalopram and other SSRIs can be used to treat hot flashes.[30]: 107
A 2009 multisite randomized controlled study found no benefit and some adverse effects in autistic children from citalopram, raising doubts whether SSRIs are effective for treating repetitive behavior in children with autism.[31]
Some research suggests citalopram interacts with cannabinoid protein-couplings in the rat brain, and this is put forward as a potential cause of some of the drug's antidepressant effect.[32]
Dosage
The defined daily dose is 20 mg by mouth or injection.[3]
Citalopram is typically taken in one dose, either in the morning or evening. It can be taken with or without food. Its absorption does not increase when taken with food,[33] but doing so can help prevent nausea. Nausea is often caused when the 5HT3 receptors actively absorb free serotonin, as this receptor is present within the digestive tract.[34] The 5HT3 receptors stimulate vomiting. This side effect, if present, should subside as the body adjusts to the medication.
Side effects
Sexual dysfunction is often a side effect with SSRIs. Specifically, common side effects include difficulty becoming aroused, lack of interest in sex, and anorgasmia (trouble achieving orgasm). One study showed, however, when remission of major depressive disorder is achieved, quality of life and sexual satisfaction is reported to be higher in spite of sexual side effects.[35]
Citalopram theoretically causes side effects by increasing the concentration of serotonin in other parts of the body (e.g., the intestines). Other side effects, such as increased apathy and emotional flattening, may be caused by the decrease in dopamine release associated with increased serotonin. Citalopram is also a mild antihistamine, which may be responsible for some of its sedating properties.[30]: 104
Common side effects of citalopram include drowsiness, insomnia, nausea, weight changes (usually weight gain), increase in appetite, vivid dreaming, frequent urination, decreased sex drive, anorgasmia, dry mouth,[33] increased sweating, trembling, diarrhea, excessive yawning, severe tinnitus, and fatigue. Less common side effects include bruxism, vomiting, cardiac arrhythmia, blood pressure changes, dilated pupils, anxiety, mood swings, headache, hyperactivity and dizziness. Rare side effects include convulsions, hallucinations, severe allergic reactions and photosensitivity.[33] If sedation occurs, the dose may be taken at bedtime rather than in the morning. Some data suggests citalopram may cause nightmares.[36] Citalopram is associated with a higher risk of arrhythmia compared to other SSRIs.[37][38]
Withdrawal symptoms can occur when this medicine is suddenly stopped, such as paraesthesiae, sleeping problems (difficulty sleeping and intense dreams), feeling dizzy, agitated or anxious, nausea, vomiting, tremors, confusion, sweating, headache, diarrhea, palpitations, changes in emotions, irritability, and eye or eyesight problems. Treatment with citalopram should be reduced gradually when treatment is finished.
Citalopram and other SSRIs can induce a mixed state, especially in those with undiagnosed bipolar disorder.[30]: 105
Sexual dysfunction
Some people experience persistent sexual side effects after they stop taking SSRIs.[39] This is known as Post-SSRI Sexual Dysfunction (PSSD). Common symptoms in these cases include genital anesthesia, erectile dysfunction, anhedonia, decreased libido, premature ejaculation, vaginal lubrication issues, and nipple insensitivity in women. The prevalence of PSSD is unknown, and there is no established treatment.[40]
Abnormal heart rhythm
In August 2011, the FDA announced, “Citalopram causes dose-dependent QT interval prolongation. Citalopram should no longer be prescribed at doses greater than 40 mg per day”.[41] A further clarification issued in March 2012 restricted the maximum dose to 20 mg for subgroups of patients, including those older than 60 years and those taking an inhibitor of cytochrome P450 2C19.7.[42]
Endocrine effects
As with other SSRIs, citalopram can cause an increase in serum prolactin level.[43] Citalopram has no significant effect on insulin sensitivity in women of reproductive age[44] and no changes in glycaemic control were seen in another trial.[28]
Exposure in pregnancy
Antidepressant exposure (including citalopram) during pregnancy is associated with shorter duration of gestation (by three days), increased risk of preterm delivery (by 55%), lower birth weight (by 75 g), and lower Apgar scores (by <0.4 points). Antidepressant exposure is not associated with an increased risk of spontaneous abortion.[45] It is uncertain whether there is an increased prevalence of septal heart defects among children whose mothers were prescribed an SSRI in early pregnancy.[46][47]
Interactions
Citalopram should not be taken with St John's wort, tryptophan or 5-HTP as the resulting drug interaction could lead to serotonin syndrome.[48] With St John's wort, this may be caused by compounds in the plant extract reducing the efficacy of the hepatic cytochrome P450 enzymes that process citalopram.[49] It has also been suggested that such compounds, including hypericin, hyperforin and flavonoids, could have SSRI-mimetic effects on the nervous system, although this is still subject to debate.[50] One study found that Hypericum extracts had similar effects in treating moderate depression as citalopram, with fewer side effects.[51]
Tryptophan and 5-HTP are precursors to serotonin.[52] When taken with an SSRI, such as citalopram, this can lead to levels of serotonin that can be lethal. This may also be the case when SSRIs are taken with SRAs (serotonin releasing agents) such as in the case of MDMA. It is possible that SSRIs could reduce the effects associated due to an SRA, since SSRIs stop the reuptake of Serotonin by blocking SERT. This would allow less serotonin in and out of the transporters, thus decreasing the likelihood of neurotoxic effects. However, these concerns are still disputed as the exact pharmacodynamic effects of citalopram and MDMA have yet to be fully identified.
SSRIs, including citalopram, can increase the risk of bleeding, especially when coupled with aspirin, NSAIDs, warfarin, or other anticoagulants.[33] Citalopram is contraindicated in individuals taking MAOIs, owing to a potential for serotonin syndrome.
Taking citalopram with omeprazole may cause higher blood levels of citalopram. This is a potentially dangerous interaction, so dosage adjustments may be needed or alternatives may be prescribed.[53][54]
SSRI discontinuation syndrome has been reported when treatment is stopped. It includes sensory, gastrointestinal symptoms, dizziness, lethargy, and sleep disturbances, as well as psychological symptoms such as anxiety/agitation, irritability, and poor concentration.[55] Electric shock-like sensations are typical for SSRI discontinuation.[56] Tapering off citalopram therapy, as opposed to abrupt discontinuation, is recommended in order to diminish the occurrence and severity of discontinuation symptoms. Some doctors choose to switch a patient to Prozac (fluoxetine) when discontinuing citalopram as fluoxetine has a much longer half-life (i.e. stays in the body longer compared to citalopram). This may avoid many of the severe withdrawal symptoms associated with citalopram discontinuation. This can be done either by administering a single 20 mg dose of fluoxetine or by beginning on a low dosage of fluoxetine and slowly tapering down. Either of these prescriptions may be written in liquid form to allow a very slow and gradual tapering down in dosage. Alternatively, a patient wishing to stop taking citalopram may visit a compounding pharmacy where his or her prescription may be re-arranged into progressively smaller dosages.
Overdose
Overdosage may result in vomiting, sedation, disturbances in heart rhythm, dizziness, sweating, nausea, tremor, and rarely amnesia, confusion, coma, or convulsions.[30]: 105 Overdose deaths have occurred, sometimes involving other drugs, but also with citalopram as the sole agent. Citalopram and N-desmethylcitalopram may be quantified in blood or plasma to confirm a diagnosis of poisoning in hospitalized patients or to assist in a medicolegal death investigation. Blood or plasma citalopram concentrations are usually in a range of 50-400 μg/l in persons receiving the drug therapeutically, 1000–3000 μg/l in patients who survive acute overdosage and 3–30 mg/l in those who do not survive.[57][58][59] It is the most dangerous of SSRIs in overdose.[60]
Suicidality
In the United States, citalopram carries a boxed warning stating it may increase suicidal thinking and behavior in those under age 24.[33]
Interactions
Citalopram is considered safe and well tolerated in the therapeutic dose range. Distinct from some other agents in its class, it exhibits linear pharmacokinetics and minimal drug interaction potential, making it a better choice for the elderly or comorbid patients.[61]
Stereochemistry
Citalopram has one stereocenter, to which a 4-fluoro phenyl group and an N, N-dimethyl-3-aminopropyl group bind. As a result of this chirality, the molecule exists in (two) enantiomeric forms (mirror images). They are termed S-(+)-citalopram and R-(–)-citalopram.
-citalopram-3D-sticks.png.webp) |
-citalopram-3D-sticks.png.webp) |
 |
-citalopram.svg.png.webp) |
| (S)-(+)-citalopram | (R)-(–)-citalopram |
Citalopram is sold as a racemic mixture, consisting of 50% (R)-(−)-citalopram and 50% (S)-(+)-citalopram. Only the (S)-(+) enantiomer has the desired antidepressant effect.[62] Lundbeck now markets the (S)-(+) enantiomer, the generic name of which is escitalopram. Whereas citalopram is supplied as the hydrobromide, escitalopram is sold as the oxalate salt (hydrooxalate).[33] In both cases, the salt forms of the amine make these otherwise lipophilic compounds water-soluble.
Metabolism
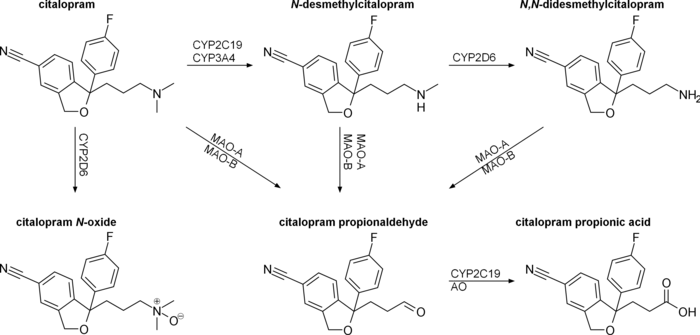
Citalopram is metabolized in the liver mostly by CYP2C19, but also by CYP3A4 and CYP2D6. Metabolites desmethylcitalopram and didesmethylcitalopram are significantly less energetic and their contribution to the overall action of citalopram is negligible. The half-life of citalopram is about 35 hours. Approximately 80% is cleared by the liver and 20% by the kidneys.[63] The elimination process is slower in the elderly and in patients with liver or kidney failure. With once-daily dosing, steady plasma concentrations are achieved in about a week. Potent inhibitors of CYP2C19 and 3A4 might decrease citalopram clearance.[64] Tobacco smoke exposure was found to inhibit the biotransformation of citalopram in animals, suggesting that the elimination rate of citalopram is decreased after tobacco smoke exposure. After intragastric administration, the half-life of the racemic mixture of citalopram was increased by about 287%.[65]
Pharmacology
| Receptor | Ki (nM) |
|---|---|
| SERT | 1.6 |
| NET | 6190 |
| 5-HT2C | 617 |
| α1 |
1211 |
| M1 | 1430 |
| H1 | 283 |
History
Citalopram was first synthesized in 1972 by chemist Klaus Bøgesø and his research group at the pharmaceutical company Lundbeck and was first marketed in 1989 in Denmark. It was first marketed in the US in 1998.[69] The patent expired in 2003, allowing other companies to legally produce generic versions.
Society and culture
Cost
In the United Kingdom, a typical dose costs the NHS less than 20 GBP per month.[6] In the United States, it costs 50 to 100 USD per month as of 2016.[7] In 2017, it was the 26th most commonly prescribed medication in the United States, with more than 24 million prescriptions.[8][9]
.svg.png.webp) Citalopram costs (US)
Citalopram costs (US).svg.png.webp) Citalopram prescriptions (US)
Citalopram prescriptions (US)
Brand names
Citalopram is sold under these brand-names:
- Akarin (Denmark, Nycomed)
- C Pram S (India)
- Celapram (Australia,[70] New Zealand),
- Celexa (U.S. and Canada, Forest Laboratories, Inc.)
- Celica (Australia)[70]
- Ciazil (Australia,[70] New Zealand)
- Cilate (South Africa)
- Cilift (South Africa)
- Cimal (South America, by Roemmers and Recalcine)
- Cipralex (South Africa)
- Cipram (Denmark, Turkey, H. Lundbeck A/S)
- Cipramil (Australia,[70] Brazil, Belgium, Chile, Finland, Germany, Netherlands, Iceland, Ireland, Israel, New Zealand, Norway, Russia, South Africa, Sweden, United Kingdom)
- Cipraned, Cinapen (Greece)
- Ciprapine (Ireland)
- Ciprotan (Ireland)
- Citabax, Citaxin (Poland)
- Cital (Poland)
- Citalec (Czech Republic, Slovakia)
- Citalex (Iran, Serbia)
- Citalo (Australia,[70] Egypt, Pakistan)
- Citalopram (Canada, Denmark, Finland, Germany, Ireland, New Zealand, Spain, Sweden, Switzerland, United Kingdom, USA)
- Citol (Russia)
- Citox (Mexico)
- Citrol (Europe and Australia)[70]
- Citta (Brazil)
- Dalsan (Eastern Europe)
- Denyl (Brazil)
- Elopram (Italy)
- Estar (Pakistan)
- Humorup (Argentina)
- Humorap (Peru, Bolivia)
- Lopraxer (Greece)[71]
- Oropram (Iceland, Actavis),
- Opra (Russia)
- Pram (Russia)
- Pramcit (Pakistan)
- Procimax (Brazil)
- Recital (Israel, Thrima Inc. for Unipharm Ltd.)
- Sepram (Finland)
- Seropram (various European countries, including Czech Republic)
- Szetalo (India)
- Talam (Europe and Australia)[70]
- Temperax (Argentina, Chile, Peru)
- Vodelax (Turkey)
- Zentius (South America, by Roemmers and Recalcine)
- Zetalo (India)
- Cipratal (Kuwait, GCC)
- Zylotex (Portugal)[72]
European Commission fine
On 19 June 2013, the European Commission imposed a fine of €93.8 million on the Danish pharmaceutical company Lundbeck, plus a total of €52.2 million on several generic pharmaceutical-producing companies. This was in response to Lundbeck entering an agreement with the companies to delay their sales of generic citalopram after Lundbeck's patent on the drug had expired, thus reducing competition in breach of European antitrust law.[73]
References
- ↑ "Citalopram". Merriam-Webster Dictionary.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Citalopram Hydrobromide Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 4 December 2020. Retrieved 9 September 2020.
- ↑ "Citalopram (Celexa) Use During Pregnancy". Drugs.com. Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- 1 2 3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 361. ISBN 9780857113382.
- 1 2 Tarascon Pharmacopoeia 2017 Professional Desk Reference Edition. Jones & Bartlett Learning. 2016. p. 440. ISBN 9781284118957. Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Citalopram Drug Usage Statistics". ClinCalc. 23 December 2019. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ↑ See p410 of "National Clinical Practice Guideline 90. Depression: The treatment and management of depression in adults, updated edition (2010)". National Institute for Health and Clinical Excellence (UK). Archived from the original on 15 December 2017. Retrieved 27 November 2016.
- ↑ Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C (February 2009). "Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis". Lancet. 373 (9665): 746–58. doi:10.1016/S0140-6736(09)60046-5. PMID 19185342.
- ↑ Cipriani A, Purgato M, Furukawa TA, Trespidi C, Imperadore G, Signoretti A, Churchill R, Watanabe N, Barbui C (July 2012). Cipriani A (ed.). "Citalopram versus other anti-depressive agents for depression". The Cochrane Database of Systematic Reviews. 7 (7): CD006534. doi:10.1002/14651858.CD006534.pub2. PMC 4204633. PMID 22786497.
- ↑ Cohen D (2007). "Should the use of selective serotonin reuptake inhibitors in child and adolescent depression be banned?". Psychotherapy and Psychosomatics. 76 (1): 5–14. doi:10.1159/000096360. PMID 17170559.
- ↑ Carandang C, Jabbal R, Macbride A, Elbe D (November 2011). "A review of escitalopram and citalopram in child and adolescent depression". Journal of the Canadian Academy of Child and Adolescent Psychiatry. 20 (4): 315–24. PMC 3222577. PMID 22114615.
- ↑ Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych (Office for Registration of Medicinal Products, Medical Devices and Biocides) "Archived copy" (PDF). Archived from the original (PDF) on 5 November 2013. Retrieved 24 September 2013.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Hellerstein DJ, Batchelder S, Miozzo R, Kreditor D, Hyler S, Gangure D, Clark J (May 2004). "Citalopram in the treatment of dysthymic disorder". International Clinical Psychopharmacology. 19 (3): 143–8. doi:10.1097/00004850-200405000-00004. PMID 15107656.
- ↑ Poore J. "Celexa (citalopram hydrobromide)". Crazy Meds. Archived from the original on 14 August 2011. Retrieved 10 July 2010.
- ↑ Varia I, Rauscher F (May 2002). "Treatment of generalized anxiety disorder with citalopram". International Clinical Psychopharmacology. 17 (3): 103–7. doi:10.1097/00004850-200205000-00002. PMID 11981350.
- ↑ Stein DJ, Montgomery SA, Kasper S, Tanghoj P (November 2001). "Predictors of response to pharmacotherapy with citalopram in obsessive-compulsive disorder". International Clinical Psychopharmacology. 16 (6): 357–61. doi:10.1097/00004850-200111000-00007. PMID 11712625.
- ↑ Pallanti S, Quercioli L, Koran LM (September 2002). "Citalopram intravenous infusion in resistant obsessive-compulsive disorder: an open trial". The Journal of Clinical Psychiatry. 63 (9): 796–801. doi:10.4088/JCP.v63n0908. PMID 12363120.
- ↑ Atmaca M, Kuloglu M, Tezcan E, Unal A (December 2002). "Efficacy of citalopram and moclobemide in patients with social phobia: some preliminary findings". Human Psychopharmacology. 17 (8): 401–5. doi:10.1002/hup.436. PMID 12457375.
- ↑ Armenteros JL, Lewis JE (May 2002). "Citalopram treatment for impulsive aggression in children and adolescents: an open pilot study". Journal of the American Academy of Child and Adolescent Psychiatry. 41 (5): 522–9. doi:10.1097/00004583-200205000-00009. PMID 12014784.
- ↑ Reist C, Nakamura K, Sagart E, Sokolski KN, Fujimoto KA (January 2003). "Impulsive aggressive behavior: open-label treatment with citalopram". The Journal of Clinical Psychiatry. 64 (1): 81–5. doi:10.4088/jcp.v64n0115. PMID 12590628.
- ↑ Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, Marin R, Jacob NJ, Huber KA, Kastango KB, Chew ML (March 2002). "Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients". The American Journal of Psychiatry. 159 (3): 460–5. doi:10.1176/appi.ajp.159.3.460. PMID 11870012.
- ↑ Tosto G, Talarico G, Lenzi GL, Bruno G (September 2008). "Effect of citalopram in treating hypersexuality in an Alzheimer's disease case". Neurological Sciences. 29 (4): 269–70. doi:10.1007/s10072-008-0979-1. PMID 18810603.
- ↑ Marjoribanks J, Brown J, O'Brien PM, Wyatt K (June 2013). "Selective serotonin reuptake inhibitors for premenstrual syndrome". The Cochrane Database of Systematic Reviews (6): CD001396. doi:10.1002/14651858.CD001396.pub3. PMC 7073417. PMID 23744611.
- ↑ Tiihonen J, Ryynänen OP, Kauhanen J, Hakola HP, Salaspuro M (January 1996). "Citalopram in the treatment of alcoholism: a double-blind placebo-controlled study". Pharmacopsychiatry. 29 (1): 27–9. doi:10.1055/s-2007-979538. PMID 8852531.
- 1 2 Sindrup SH, Bjerre U, Dejgaard A, Brøsen K, Aaes-Jørgensen T, Gram LF (November 1992). "The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy". Clinical Pharmacology and Therapeutics. 52 (5): 547–52. doi:10.1038/clpt.1992.183. PMID 1424428.
- ↑ Rampello L, Alvano A, Chiechio S, Malaguarnera M, Raffaele R, Vecchio I, Nicoletti F (2004). "Evaluation of the prophylactic efficacy of amitriptyline and citalopram, alone or in combination, in patients with comorbidity of depression, migraine, and tension-type headache". Neuropsychobiology. 50 (4): 322–8. doi:10.1159/000080960. PMID 15539864.
- 1 2 3 4 Stahl SM (2011). The Prescriber's Guide (Stahl's Essential Psychopharmacology). Cambridge, UK: Cambridge University Press. ISBN 978-0-521-17364-3.
- ↑ King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, Donnelly CL, Anagnostou E, Dukes K, Sullivan L, Hirtz D, Wagner A, Ritz L (June 2009). "Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism". Archives of General Psychiatry. 66 (6): 583–90. doi:10.1001/archgenpsychiatry.2009.30. PMC 4112556. PMID 19487623.
- Lay summary in: "Study finds antidepressant doesn't help autistic children". Los Angeles Times. 2 June 2009.
- ↑ Hesketh SA, Brennan AK, Jessop DS, Finn DP (May 2008). "Effects of chronic treatment with citalopram on cannabinoid and opioid receptor-mediated G-protein coupling in discrete rat brain regions". Psychopharmacology. 198 (1): 29–36. doi:10.1007/s00213-007-1033-3. PMID 18084745.
- 1 2 3 4 5 6 "Celexa (citalopram hydrobromide) Tablets/Oral Solution" (PDF). Prescribing Information. Forest Laboratories, Inc. Archived (PDF) from the original on 21 October 2014. Retrieved 20 October 2012.
- ↑ Rang HP (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 187. ISBN 978-0-443-07145-4.
- ↑ Ishak WW, Christensen S, Sayer G, Ha K, Li N, Miller J, Nguyen JM, Cohen RM (March 2013). "Sexual satisfaction and quality of life in major depressive disorder before and after treatment with citalopram in the STAR*D study". The Journal of Clinical Psychiatry. 74 (3): 256–61. doi:10.4088/JCP.12m07933. PMID 23561231.
- ↑ Arora G, Sandhu G, Fleser C (Spring 2012). "Citalopram and nightmares". The Journal of Neuropsychiatry and Clinical Neurosciences. 24 (2): E43. doi:10.1176/appi.neuropsych.11040096. PMID 22772700.
- ↑ Qirjazi E, McArthur E, Nash DM, Dixon SN, Weir MA, Vasudev A, Jandoc R, Gula LJ, Oliver MJ, Wald R, Garg AX (11 August 2016). "Risk of Ventricular Arrhythmia with Citalopram and Escitalopram: A Population-Based Study". PLOS ONE. 11 (8): e0160768. doi:10.1371/journal.pone.0160768. PMC 4981428. PMID 27513855.
- ↑ Girardin FR, Gex-Fabry M, Berney P, Shah D, Gaspoz JM, Dayer P (December 2013). "Drug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG Screening Outcome in Psychiatry study". The American Journal of Psychiatry. 170 (12): 1468–76. doi:10.1176/appi.ajp.2013.12060860. PMID 24306340.
- ↑ American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. pp. 449. ISBN 9780890425558.
- ↑ Bala A, Nguyen HM, Hellstrom WJ (January 2018). "Post-SSRI Sexual Dysfunction: A Literature Review". Sexual Medicine Reviews. 6 (1): 29–34. doi:10.1016/j.sxmr.2017.07.002. PMID 28778697.
- ↑ "Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide)". Safety Communication. United States Food and Drug Administration. 24 August 2011.
- ↑ "FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses". Drug Safety and Availability. United States Food and Drug Administration. 22 May 2012
- ↑ Trenque T, Herlem E, Auriche P, Dramé M (December 2011). "Serotonin reuptake inhibitors and hyperprolactinaemia: a case/non-case study in the French pharmacovigilance database". Drug Safety. 34 (12): 1161–6. doi:10.2165/11595660-000000000-00000. PMID 22077504.
- ↑ Kauffman RP, Castracane VD, White DL, Baldock SD, Owens R (September 2005). "Impact of the selective serotonin reuptake inhibitor citalopram on insulin sensitivity, leptin and basal cortisol secretion in depressed and non-depressed euglycemic women of reproductive age". Gynecological Endocrinology. 21 (3): 129–37. doi:10.1080/09513590500216800. PMID 16335904.
- ↑ Ross LE, Grigoriadis S, Mamisashvili L, Vonderporten EH, Roerecke M, Rehm J, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A (April 2013). "Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis". JAMA Psychiatry. 70 (4): 436–43. doi:10.1001/jamapsychiatry.2013.684. PMID 23446732.
- ↑ Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH (September 2009). "Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study". BMJ. 339 (sep23 1): b3569. doi:10.1136/bmj.b3569. PMC 2749925. PMID 19776103.
- ↑ Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, Mogun H, Levin R, Kowal M, Setoguchi S, Hernández-Díaz S (June 2014). "Antidepressant use in pregnancy and the risk of cardiac defects". The New England Journal of Medicine. 370 (25): 2397–407. doi:10.1056/NEJMoa1312828. PMC 4062924. PMID 24941178.
- ↑ Karch AM (2006). Lippincott's Nursing Drug Guide. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-1-58255-436-5.
- ↑ "Interactions with St John's wort preparations". Prescriber Update Articles. New Zealand Medicines and Medical Devices Safety Authority. 2000. Archived from the original on 3 February 2009. Retrieved 27 February 2009.
- ↑ "St. John's wort". Medical Reference. University of Maryland Medical Center. 2011. Archived from the original on 5 March 2009. Retrieved 27 February 2009.
- ↑ Gastpar M, Singer A, Zeller K (March 2006). "Comparative efficacy and safety of a once-daily dosage of hypericum extract STW3-VI and citalopram in patients with moderate depression: a double-blind, randomised, multicentre, placebo-controlled study". Pharmacopsychiatry. 39 (2): 66–75. doi:10.1055/s-2006-931544. PMID 16555167.
- ↑ "The History of Tryptophan, Serotonin and 5-HTP". www.oxfordvitality.co.uk. Archived from the original on 23 July 2018. Retrieved 22 July 2018.
- ↑ "Drug interactions between Celexa and omeprazole". Drugs.com. Archived from the original on 1 February 2014. Retrieved 28 January 2014.
- ↑ "citalopram (Rx) - Celexa". Medscape. Archived from the original on 5 December 2013. Retrieved 28 January 2014.
- ↑ Warner CH, Bobo W, Warner C, Reid S, Rachal J (August 2006). "Antidepressant discontinuation syndrome". American Family Physician. 74 (3): 449–56. PMID 16913164.
- ↑ Prakash O, Dhar V (June 2008). "Emergence of electric shock-like sensations on escitalopram discontinuation". Journal of Clinical Psychopharmacology. 28 (3): 359–60. doi:10.1097/JCP.0b013e3181727534. PMID 18480703.
- ↑ Research, Center for Drug Evaluation and (24 August 2011). "Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide)". Safety Communication. United States Food and Drug Administration. Archived from the original on 10 October 2020. Retrieved 16 December 2019.
- ↑ Personne M, Sjöberg G, Persson H (1997). "Citalopram overdose--review of cases treated in Swedish hospitals". Journal of Toxicology. Clinical Toxicology. 35 (3): 237–40. doi:10.3109/15563659709001206. PMID 9140316.
- ↑ Luchini D, Morabito G, Centini F (December 2005). "Case report of a fatal intoxication by citalopram". The American Journal of Forensic Medicine and Pathology. 26 (4): 352–4. doi:10.1097/01.paf.0000188276.33030.dd. PMID 16304470.
- ↑ Taylor D, Paton C, Kapur S (2012). The Maudsley Prescribing Guidelines in Psychiatry (Taylor, The Maudsley Prescribing Guidelines). Hoboken, NJ, USA: Wiley-Blackwell. p. 588. ISBN 9780470979693.
- ↑ Keller MB (December 2000). "Citalopram therapy for depression: a review of 10 years of European experience and data from U.S. clinical trials". The Journal of Clinical Psychiatry. 61 (12): 896–908. doi:10.4088/JCP.v61n1202. PMID 11206593.
- ↑ Lepola U, Wade A, Andersen HF (May 2004). "Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorder". International Clinical Psychopharmacology. 19 (3): 149–55. doi:10.1097/00004850-200405000-00005. PMID 15107657.
- ↑ "Citalopram". DrugBank. 17 August 2016. Archived from the original on 12 February 2017. Retrieved 12 February 2017.
- ↑ Final Labelling Citalopram https://www.fda.gov/ohrms/dockets/ac/04/briefing/4006b1_07_celexa-label.pdf Wayback Machine at the Wayback Machine (archived 16 May 2017)
- ↑ Majcherczyk J, Kulza M, Senczuk-Przybylowska M, Florek E, Jawien W, Piekoszewski W (February 2012). "Influence of tobacco smoke on the pharmacokinetics of citalopram and its enantiomers". Journal of Physiology and Pharmacology. 63 (1): 95–100. PMID 22460466.
- ↑ Sangkuhl K, Klein TE, Altman RB (November 2011). "PharmGKB summary: citalopram pharmacokinetics pathway". Pharmacogenetics and Genomics. 21 (11): 769–72. doi:10.1097/FPC.0b013e328346063f. PMC 3349993. PMID 21546862.
- ↑ Hiemke C, Härtter S (January 2000). "Pharmacokinetics of selective serotonin reuptake inhibitors". Pharmacology & Therapeutics. 85 (1): 11–28. doi:10.1016/s0163-7258(99)00048-0. PMID 10674711.
- ↑ Owens JM, Knight DL, Nemeroff CB (Jul–Aug 2002). "[Second generation SSRIS: human monoamine transporter binding profile of escitalopram and R-fluoxetine]". L'Encephale. 28 (4): 350–5. PMID 12232544.
- ↑ Benjamin Rawe and Paul May for Molecule of the Month. 2009 Citalopram: A new treatment for depression Citalopram - Molecule of the Month 2009 - HTML-only version at the Wayback Machine (archived 1 March 2017) Page accessed 16 February 2015.
- 1 2 3 4 5 6 7 Health, Australian Government Department of. "Pharmaceutical Benefits Scheme (PBS) - Citalopram". Australian Government. Archived from the original on 11 September 2014. Retrieved 10 September 2014.
- ↑ "Lopraxer". Drugs.com. Archived from the original on 12 April 2020. Retrieved 12 April 2020.
- ↑ "Citalopram". International. Drugs.com. Archived from the original on 16 September 2018. Retrieved 23 January 2018.
- ↑ "Antitrust: Commission fines Lundbeck and other pharma companies for delaying market entry of generic medicines" (Press release). Brussels: European Union. 19 June 2013. Archived from the original on 22 June 2013. Retrieved 20 June 2013.
External links
| External sites: |
|
|---|---|
| Identifiers: |