Cericlamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral[1] |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 8 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
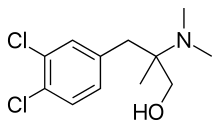
| Formula | C12H17Cl2NO |
| Molar mass | 262.17 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Cericlamine (INN; developmental code JO-1017) is a potent and moderately selective serotonin reuptake inhibitor (SSRI) of the amphetamine family (specifically, a derivative of phentermine, and closely related to chlorphentermine, a highly selective serotonin releasing agent) that was investigated as an antidepressant for the treatment of depression, anxiety disorders, and anorexia nervosa by Jouveinal but did not complete development and was never marketed.[1][2][3][4] It reached phase III clinical trials in 1996 before development was discontinued in 1999.[5]
Synthesis
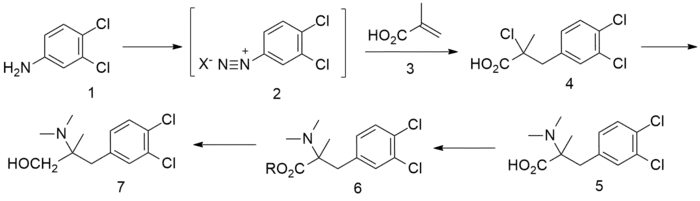
Arylation of methacrylic acid with a diazonium salt of 3,4-dichloroaniline (or 3,4-dichloro-benzenediazonium salt), is carried out according to the Meerwein reaction catalysed by a metallic halide. For the next step, the halide is displaced by dimethylamine, then esterification is performed, followed by reduction with a metal hydride.
See also
References
- 1 2 3 Darcourt G, Tessera M, Lesaunier R, Engrand P, Scherrer B, Dreyfus J, Bogaievsky Y (1992). "A Multicentre Double-Blind, Placebo-Controlled Dose-Finding Study with Cericlamine in Major Depression". Clinical Neuropharmacology. 15: 176B. doi:10.1097/00002826-199202001-00339. ISSN 0362-5664. S2CID 57983762.
- ↑ Crow S, Brown E (March 2003). "Investigational drugs for eating disorders". Expert Opinion on Investigational Drugs. 12 (3): 491–9. doi:10.1517/13543784.12.3.491. PMID 12605570. S2CID 25463729.
- ↑ Ramesh N. Patel (3 January 2000). Stereoselective Biocatalysis. CRC Press. pp. 48–. ISBN 978-0-8247-8282-5.
- ↑ Tang LC, Tang SJ (6 December 2012). Neurochemistry in Clinical Application. Springer Science & Business Media. pp. 81–. ISBN 978-1-4615-1857-0.
- ↑ "Cericlamine". AdisInsight. Springer Nature Switzerland AG. Retrieved 13 January 2016.
- ↑ US 6121491, Nicolas M, Laboue B, Depernet D, "Process for the preparation of (+/-)3-(3,4-dichlorophenyl)-2-dimethylamino-2-methylpropan-1-OL or cericlamine", issued 19 September 200, assigned to Warner Lambert