Dexfenfluramine
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a682088 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 36% |
| Elimination half-life | 17–20 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
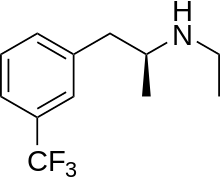
| Formula | C12H16F3N |
| Molar mass | 231.262 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Dexfenfluramine, marketed as dexfenfluramine hydrochloride under the name Redux, is a serotonergic anorectic drug: it reduces appetite by increasing the amount of extracellular serotonin in the brain.[1] It is the d-enantiomer of fenfluramine and is structurally similar to amphetamine, but lacks any psychologically stimulating effects.
Dexfenfluramine was, for some years in the mid-1990s, approved by the United States Food and Drug Administration for the purposes of weight loss. However, following multiple concerns about the cardiovascular side-effects of the drug,[1] the FDA withdrew the approval in 1997.[2] After it was removed in the US, dexfenfluramine was also pulled out in other global markets. It was later superseded by sibutramine, which, although initially considered a safer alternative to both dexfenfluramine and fenfluramine, was likewise removed from the US market in 2010.[3]
The drug was developed by Interneuron Pharmaceuticals, a company co-founded by Richard Wurtman, aimed at marketing discoveries by Massachusetts Institute of Technology scientists.[4] Interneuron licensed the patent to Wyeth-Ayerst Laboratories.[5] Although at the time of its release, some optimism prevailed that it might herald a new approach, there remained some reservations amongst neurologists, twenty-two of whom petitioned the FDA to delay approval. Their concern was based on the work of George A. Ricaurte, whose techniques and conclusions were later questioned.[6]
See also
References
- 1 2 Fox SI (2011). Human Physiology (Twelfth ed.). McGraw Hill. p. 665.
- ↑ FDA 15 September 1997. FDA Announces Withdrawal Fenfluramine and Dexfenfluramine (Fen-Phen)
- ↑ "Abbott Pulls Diet Drug Meridia Off US Shelves". The Wall Street Journal. 8 October 2010. Archived from the original on 23 October 2010.
- ↑ Lemonick MD, Dowell W, Nash JM, Ramirez A, Reid B, Ressner J (23 September 1996). "The New Miracle Drug?". Time. Archived from the original on 6 November 2010. Retrieved 3 October 2010.
- ↑ Lemonick MD, Nash JM, Park A, Thompson D (29 September 1997). "The Mood Molecule". Time. Archived from the original on 3 October 2008. Retrieved 4 October 2010.
- ↑ "DEA Accedes to Ecstasy Test". Wired. 2 March 2004.
External links
- Drug description
- Dexfenfluramine hydrochloride
- Questions and Answers about Withdrawal of Fenfluramine (Pondimin) and Dexfenfluramine (Redux)
- Frontline: Dangerous prescriptions - Interview with Leo Lutwak, M.D. in which he discuses the side effects of fenfluramine, its successor Redux, and the Fen-Phen combination
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|