Sarizotan
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H21FN2O |
| Molar mass | 348.421 g·mol−1 |
| 3D model (JSmol) | |
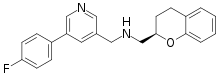
SMILES
| |
Sarizotan (EMD-128,130) is a selective 5-HT1A receptor agonist and D2 receptor antagonist,[1] which has antipsychotic effects,[2][3] and has also shown efficacy in reducing dyskinesias resulting from long-term anti-Parkinsonian treatment with levodopa.[4][5][6][7]
In June 2006, the developer Merck KGaA announced that the development of sarizotan was discontinued, after two sarizotan Phase III studies (PADDY I, PADDY II) failed to meet the primary efficacy endpoint and neither the Phase II findings nor the results from preclinical studies could be confirmed. No statistically significant difference of the primary target variable between sarizotan and placebo could be demonstrated.[8][9]
See also
References
- ↑ Rabiner EA, Gunn RN, Wilkins MR, Sedman E, Grasby PM (September 2002). "Evaluation of EMD 128 130 occupancy of the 5-HT1A and the D2 receptor: a human PET study with [11C]WAY-100635 and [11C]raclopride". Journal of Psychopharmacology. 16 (3): 195–9. doi:10.1177/026988110201600301. PMID 12236624. S2CID 12943982.
- ↑ Assié MB, Ravailhe V, Faucillon V, Newman-Tancredi A (October 2005). "Contrasting contribution of 5-hydroxytryptamine 1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain". The Journal of Pharmacology and Experimental Therapeutics. 315 (1): 265–72. doi:10.1124/jpet.105.087163. PMID 15987834. S2CID 9262233.
- ↑ Auclair AL, Galinier A, Besnard J, Newman-Tancredi A, Depoortère R (July 2007). "Putative antipsychotics with pronounced agonism at serotonin 5-HT1A and partial agonist activity at dopamine D2 receptors disrupt basal PPI of the startle reflex in rats". Psychopharmacology. 193 (1): 45–54. doi:10.1007/s00213-007-0762-7. PMID 17393144. S2CID 23379049.
- ↑ Bibbiani F, Oh JD, Chase TN (November 2001). "Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models". Neurology. 57 (10): 1829–34. doi:10.1212/wnl.57.10.1829. PMID 11723272. S2CID 24836560.
- ↑ Bartoszyk GD, Van Amsterdam C, Greiner HE, Rautenberg W, Russ H, Seyfried CA (February 2004). "Sarizotan, a serotonin 5-HT1A receptor agonist and dopamine receptor ligand. 1. Neurochemical profile". Journal of Neural Transmission. 111 (2): 113–26. doi:10.1007/s00702-003-0094-7. PMID 14767715. S2CID 25419329.
- ↑ Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, Chase TN (August 2005). "Effects of serotonin 5-HT1A agonist in advanced Parkinson's disease". Movement Disorders. 20 (8): 932–6. doi:10.1002/mds.20370. PMID 15791634. S2CID 46098045.
- ↑ Grégoire L, Samadi P, Graham J, Bédard PJ, Bartoszyk GD, Di Paolo T (July 2009). "Low doses of sarizotan reduce dyskinesias and maintain antiparkinsonian efficacy of L-Dopa in parkinsonian monkeys". Parkinsonism & Related Disorders. 15 (6): 445–52. doi:10.1016/j.parkreldis.2008.11.001. PMID 19196540.
- ↑ "Merck KGaA: Development of Sarizotan to treat Parkinson's patients will not be pursued". Ad Hoc News. 23 June 2006.
- ↑ "Merck KGaA discontinues development of Parkinson drug Sarizotan UPDATE". Forbes. 23 June 2006.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.