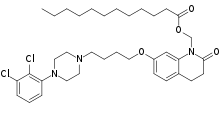
Aripiprazole lauroxil
 | |
| Clinical data | |
|---|---|
| Trade names | Aristada, Aristada Initio |
| Other names | N-Lauroyloxymethylaripiprazole; ALKS-9070; ALKS-9072; RDC-3317; Dodecanoic acid-[7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2-oxo-1(2H)-quinolinyl]methyl ester |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615048 |
| Pregnancy category |
|
| Routes of administration | Intramuscular |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.261.570 |
| Chemical and physical data | |
| Formula | C36H51Cl2N3O4 |
| Molar mass | 660.72 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Aripiprazole lauroxil, sold under the brand name Aristada, is a long-acting injectable atypical antipsychotic that was developed by Alkermes.[2][3][4] It is an N-acyloxymethyl prodrug of aripiprazole that is administered via intramuscular injection once every four to eight weeks for the treatment of schizophrenia.[2][3][4] Aripiprazole lauroxil was approved by the U.S. Food and Drug Administration (FDA) on 5 October 2015.[5][6]
Medical uses
Aripiprazole lauroxil extended release injection gained FDA approval in 2015, as a treatment for adults suffering from schizophrenia. Like any long-term acting injectable, aripiprazole lauroxil provides assurance to families and health care professionals that patients receive therapeutic medication throughout the day.[7]
Aripiprazole lauroxil is injected into the arm or buttocks of a patient by a health care professional once every four to six weeks. Aripiprazole lauroxil is a longer-lasting and injectable version of the schizophrenia pill aripiprazole, which means that the treatment is available in two doses. Aripiprazole lauroxil, along with other drugs in its family, are not approved for treatment of elderly patients with dementia-related psychosis.[7][8]
Schizophrenia
The approval of aripiprazole lauroxil from the Food and Drug Administration in 2015 was solely for the treatment of schizophrenia in adults. The ability to supplement aripiprazole lauroxil with oral supplements of aripiprazole allows for dosing flexibility, which is important for the treatment of schizophrenia, as symptoms and intensity of the disease vary greatly from patient to patient. Additionally, as in concurrence with its sister drug aripiprazole, aripiprazole lauroxil is similar in effect of typical antipsychotic drugs.[9] In the sister drug aripiprazole, side effects for patients were less frequently extrapyramidal than most antipsychotic drugs.
Side effects
The most common side effects are akathisia. According to the drug’s warning label and safety information, the side effects are large in variety.[10]
The complete list of side effects include: akathisia, Contraindication Cerebrovascular Adverse Reactions (Including Stroke), Neuroleptic Malignant Syndrome, Tardive Dyskinesia, metabolic changes, Hyperglycemia/Diabetes Mellitus, Dyslipidemia, weight gain, Orthostatic Hypotension, Leukopenia, Neutropenia, Agranulocytosis, seizures, potential for Cognitive and Motor Impairment, difficulties with body temperature regulation, Dysphagia, Injection-Site Reactions (rash, swelling, redness, irritation at the point of injection), Dystonia and pregnancy and nursing complications.[11]
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[12] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[13] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[13] Less commonly there may be a feeling of the world spinning, numbness, or muscle pains.[13] Symptoms generally resolve after a short period of time.[13]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[14] It may also result in reoccurrence of the condition that is being treated.[15] Rarely tardive dyskinesia can occur when the medication is stopped.[13]
Overdosing
The largest known case of ingestion with a known outcome involved a 1260 mg of oral aripiprazole, 42 times the recommended dose. The patient survived and fully recovered.
Common adverse reactions, reported in at least 5% of overdose cases, included vomiting, somnolence, and tremor. Other clinically important signs and symptoms of overdoses include acidosis, aggression, atrial fibrillation, bradycardia, coma, confusion, convulsion, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.[7]
Pharmacology
Mechanism of action
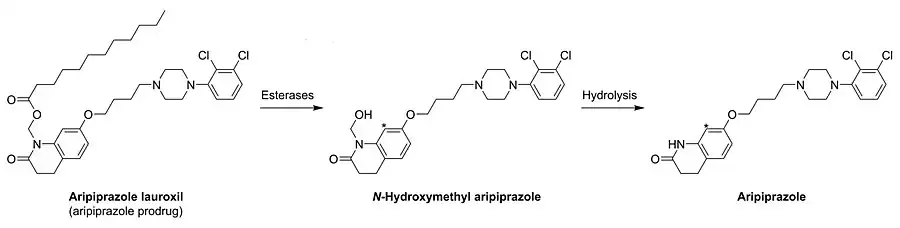
Arristada is injected into the intramuscles as an atypical antipsychotic. In one 12-week clinical trial involving 622 participants, the efficacy of extended aripiprazole was demonstrated.[8][11] Its mechanism of action is not completely known, but is thought to be converted by enzyme-mediated hydrolysis to N-hydroxymethyl aripirazole. The hydroxymethyl aripirazole is then hydrolysed to aripiprazole. Efficacy could be mediated through a combination of partial agonist activity D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. Since it is a newly approved drug by the FDA, many validation of mechanisms of action are still being studied.[11]
Pharmacodynamics
Aripiprazole exhibits high affinity for serotonin 5-HT1A, 5-HT2A receptors, dopamine D2, and dopamine D3. Moderate affinity is exhibited for serotonin 5-HT7, alpha1-adrenergic, dopamine D4, histamine H1, and serotonin re-uptake site. No affinity for cholinergic muscarinic receptors have been found.[11]
Pharmacokinetics
Aristada’s activity in the body is due to aripiprazole and also dehydro-aripiprazole. Dehydro-aripirazole has been shown to have affinities for D2 receptors. These D2 receptors have similarities to aripiprazole whereas they represent 30-40% of exposure of aripiprazole in plasma.
After five to six days of the single intramuscular injection appearance of aripiprazole in circulation, it additionally will be released for 36 days. In the fourth monthly injection, consecutive doses of Aristada will reach steady-state. With additional supplements of the oral aripiprazole at a dosage of 21 days during the first dose of Aristada, aripiprazole concentrations within 4 days can reach therapeutic levels.[11]
| Medication | Brand name | Class | Vehicle | Dosage | Tmax | t1/2 single | t1/2 multiple | logPc | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole lauroxil | Aristada | Atypical | Watera | 441–1064 mg/4–8 weeks | 24–35 days | ? | 54–57 days | 7.9–10.0 | |
| Aripiprazole monohydrate | Abilify Maintena | Atypical | Watera | 300–400 mg/4 weeks | 7 days | ? | 30–47 days | 4.9–5.2 | |
| Bromperidol decanoate | Impromen Decanoas | Typical | Sesame oil | 40–300 mg/4 weeks | 3–9 days | ? | 21–25 days | 7.9 | [16] |
| Clopentixol decanoate | Sordinol Depot | Typical | Viscoleob | 50–600 mg/1–4 weeks | 4–7 days | ? | 19 days | 9.0 | [17] |
| Flupentixol decanoate | Depixol | Typical | Viscoleob | 10–200 mg/2–4 weeks | 4–10 days | 8 days | 17 days | 7.2–9.2 | [17][18] |
| Fluphenazine decanoate | Prolixin Decanoate | Typical | Sesame oil | 12.5–100 mg/2–5 weeks | 1–2 days | 1–10 days | 14–100 days | 7.2–9.0 | [19][20][21] |
| Fluphenazine enanthate | Prolixin Enanthate | Typical | Sesame oil | 12.5–100 mg/1–4 weeks | 2–3 days | 4 days | ? | 6.4–7.4 | [20] |
| Fluspirilene | Imap, Redeptin | Typical | Watera | 2–12 mg/1 week | 1–8 days | 7 days | ? | 5.2–5.8 | [22] |
| Haloperidol decanoate | Haldol Decanoate | Typical | Sesame oil | 20–400 mg/2–4 weeks | 3–9 days | 18–21 days | 7.2–7.9 | [23][24] | |
| Olanzapine pamoate | Zyprexa Relprevv | Atypical | Watera | 150–405 mg/2–4 weeks | 7 days | ? | 30 days | – | |
| Oxyprothepin decanoate | Meclopin | Typical | ? | ? | ? | ? | ? | 8.5–8.7 | |
| Paliperidone palmitate | Invega Sustenna | Atypical | Watera | 39–819 mg/4–12 weeks | 13–33 days | 25–139 days | ? | 8.1–10.1 | |
| Perphenazine decanoate | Trilafon Dekanoat | Typical | Sesame oil | 50–200 mg/2–4 weeks | ? | ? | 27 days | 8.9 | |
| Perphenazine enanthate | Trilafon Enanthate | Typical | Sesame oil | 25–200 mg/2 weeks | 2–3 days | ? | 4–7 days | 6.4–7.2 | [25] |
| Pipotiazine palmitate | Piportil Longum | Typical | Viscoleob | 25–400 mg/4 weeks | 9–10 days | ? | 14–21 days | 8.5–11.6 | [18] |
| Pipotiazine undecylenate | Piportil Medium | Typical | Sesame oil | 100–200 mg/2 weeks | ? | ? | ? | 8.4 | |
| Risperidone | Risperdal Consta | Atypical | Microspheres | 12.5–75 mg/2 weeks | 21 days | ? | 3–6 days | – | |
| Zuclopentixol acetate | Clopixol Acuphase | Typical | Viscoleob | 50–200 mg/1–3 days | 1–2 days | 1–2 days | 4.7–4.9 | ||
| Zuclopentixol decanoate | Clopixol Depot | Typical | Viscoleob | 50–800 mg/2–4 weeks | 4–9 days | ? | 11–21 days | 7.5–9.0 | |
| Note: All by intramuscular injection. Footnotes: a = Microcrystalline or nanocrystalline aqueous suspension. b = Low-viscosity vegetable oil (specifically fractionated coconut oil with medium-chain triglycerides). c = Predicted, from PubChem and DrugBank. Sources: Main: See template. | |||||||||
Chemistry
In contrast to many other depot antipsychotics, aripiprazole lauroxil is described as a non-ester chemical modification.[26] It is specifically N-lauroyloxymethylaripiprazole.[26] However, the N-lauroyloxymethyl moiety contains a laurate ester, technically making aripiprazole lauroxil an antipsychotic ester.[27] More specifically, aripiprazole lauroxil is the laurate ester of N-hydroxymethylaripiprazole.[2] Following cleavage of the laurate ester, N-hydroxymethylaripiprazole is further metabolized to aripiprazole, making aripiprazole lauroxil a prodrug of aripiprazole with N-hydroxymethylaripiprazole as an intermediate.[27][26]
References
- ↑ "Aripiprazole Use During Pregnancy". Drugs.com. 5 February 2020. Retrieved 29 August 2020.
- 1 2 3 4 Rohde M, M Rk N, Håkansson AE, Jensen KG, Pedersen H, Dige T, J Rgensen EB, Holm R (2014). "Biological conversion of aripiprazole lauroxil - An N-acyloxymethyl aripiprazole prodrug". Results Pharma Sci. 4: 19–25. doi:10.1016/j.rinphs.2014.04.002. PMC 4050360. PMID 25756003.
- 1 2 Turncliff R, Hard M, Du Y, Risinger R, Ehrich EW (2014). "Relative bioavailability and safety of aripiprazole lauroxil, a novel once-monthly, long-acting injectable atypical antipsychotic, following deltoid and gluteal administration in adult subjects with schizophrenia". Schizophr. Res. 159 (2–3): 404–10. doi:10.1016/j.schres.2014.09.021. PMID 25266547. S2CID 1036686.
- 1 2 Meltzer HY, Risinger R, Nasrallah HA, Du Y, Zummo J, Corey L, Bose A, Stankovic S, Silverman BL, Ehrich EW (2015). "A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia". J Clin Psychiatry. 76 (8): 1085–90. doi:10.4088/JCP.14m09741. PMID 26114240.
- ↑ Citrome L (2015). "Aripiprazole Long-Acting Injectable Formulations for Schizophrenia: Aripiprazole Monohydrate and Aripiprazole Lauroxil". Expert Rev Clin Pharmacol. 9 (2): 169–86. doi:10.1586/17512433.2016.1121809. PMID 26573020. S2CID 207208248.
- ↑ "Aristada (Aripiprazole lauroxil) FDA Approval History".
- 1 2 3 "Aristada intramuscular : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD". WebMD. Retrieved 2016-04-18.
- 1 2 "New Medical Devices". Pharmacy and Therapeutics. 40 (11): 716–774. 2015-11-01. ISSN 1052-1372. PMC 4634342. PMID 26609204.
- ↑ "Aristada". Drugs.com.
- ↑ "ARISTADA (aripiprazole lauroxil) | Treatment Prescribing Information". aristada.com. Archived from the original on 2016-04-03. Retrieved 2016-04-18.
- 1 2 3 4 5 "DailyMed - ARISTADA- aripiprazole lauroxil injection, suspension, extended release". dailymed.nlm.nih.gov. Retrieved 2016-04-18.
- ↑ Joint Formulary Committee, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- 1 2 3 4 5 Haddad, Peter; Haddad, Peter M.; Dursun, Serdar; Deakin, Bill (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. p. 207–216. ISBN 9780198527480.
- ↑ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655. S2CID 6267180.
- ↑ Sacchetti, Emilio; Vita, Antonio; Siracusano, Alberto; Fleischhacker, Wolfgang (2013). Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. p. 85. ISBN 9788847026797.
- ↑ Parent M, Toussaint C, Gilson H (1983). "Long-term treatment of chronic psychotics with bromperidol decanoate: clinical and pharmacokinetic evaluation". Current Therapeutic Research. 34 (1): 1–6.
- 1 2 Jørgensen A, Overø KF (1980). "Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. III. Serum levels". Acta Psychiatrica Scandinavica. Supplementum. 279: 41–54. doi:10.1111/j.1600-0447.1980.tb07082.x. PMID 6931472.
- 1 2 Reynolds JE (1993). "Anxiolytic sedatives, hypnotics and neuroleptics.". Martindale: The Extra Pharmacopoeia (30th ed.). London: Pharmaceutical Press. pp. 364–623.
- ↑ Ereshefsky L, Saklad SR, Jann MW, Davis CM, Richards A, Seidel DR (May 1984). "Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches". The Journal of Clinical Psychiatry. 45 (5 Pt 2): 50–9. PMID 6143748.
- 1 2 Curry SH, Whelpton R, de Schepper PJ, Vranckx S, Schiff AA (April 1979). "Kinetics of fluphenazine after fluphenazine dihydrochloride, enanthate and decanoate administration to man". British Journal of Clinical Pharmacology. 7 (4): 325–31. doi:10.1111/j.1365-2125.1979.tb00941.x. PMC 1429660. PMID 444352.
- ↑ Young D, Ereshefsky L, Saklad SR, Jann MW, Garcia N (1984). Explaining the pharmacokinetics of fluphenazine through computer simulations. (Abstract.). 19th Annual Midyear Clinical Meeting of the American Society of Hospital Pharmacists. Dallas, Texas.
- ↑ Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, van Nueten JM, et al. (November 1970). "The pharmacology of fluspirilene (R 6218), a potent, long-acting and injectable neuroleptic drug". Arzneimittel-Forschung. 20 (11): 1689–98. PMID 4992598.
- ↑ Beresford R, Ward A (January 1987). "Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis". Drugs. 33 (1): 31–49. doi:10.2165/00003495-198733010-00002. PMID 3545764.
- ↑ Reyntigens AJ, Heykants JJ, Woestenborghs RJ, Gelders YG, Aerts TJ (1982). "Pharmacokinetics of haloperidol decanoate. A 2-year follow-up". International Pharmacopsychiatry. 17 (4): 238–46. doi:10.1159/000468580. PMID 7185768.
- ↑ Larsson M, Axelsson R, Forsman A (1984). "On the pharmacokinetics of perphenazine: a clinical study of perphenazine enanthate and decanoate". Current Therapeutic Research. 36 (6): 1071–88.
- 1 2 3 Correll CU, Kim E, Sliwa JK, Hamm W, Gopal S, Mathews M, Venkatasubramanian R, Saklad SR (January 2021). "Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview". CNS Drugs. 35 (1): 39–59. doi:10.1007/s40263-020-00779-5. PMC 7873121. PMID 33507525.
- 1 2 Ma Z, Zhang H, Wang Y, Tang X (April 2019). "Development and evaluation of intramuscularly administered nano/microcrystal suspension". Expert Opin Drug Deliv. 16 (4): 347–361. doi:10.1080/17425247.2019.1588248. PMID 30827123.
External links
- "Aripiprazole lauroxil". Drug Information Portal. U.S. National Library of Medicine.