Zipeprol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.432 |
| Chemical and physical data | |
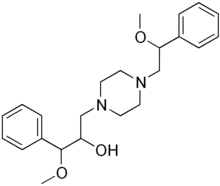
| Formula | C23H32N2O3 |
| Molar mass | 384.520 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Zipeprol is a centrally acting cough suppressant developed in France in the 1970s.[1] It is not a morphinan derivative (in contrast to codeine and dextromethorphan). Zipeprol acts as a local anaesthetic and has mucolytic, antihistamine and anticholinergic properties.[2] It is sold with several brand names such as Zinolta and Respilene. It is not available in the United States or Canada and has been discontinued in Europe. It is still available in some countries in Asia and South America.
Zipeprol has been misused in Korea, mainly for the hallucinations it produces. Such use has become an issue due to the seizures and various neurological side effects it causes at high dosages.[3]
References
- ↑ Patent US 3718650, Mauvernay RY, "New Derivatives of Substituted Piperazines", issued 1973-02-27
- ↑ Rispat G, Burgi H, Cosnier D, Duchêne-Marullaz P, Streichenberger G (April 1976). "General pharmacological properties of a new non-opiate antitussive: zipeprol (3024 CERM). I. Action on respiratory function and acute toxicity". Arzneimittel-Forschung. 26 (4): 523–30. PMID 8057.
- ↑ Chung H, Park M, Hahn E, Choi H, Choi H, Lim M (October 2004). "Recent trends of drug abuse and drug-associated deaths in Korea". Annals of the New York Academy of Sciences. 1025 (1): 458–64. Bibcode:2004NYASA1025..458C. doi:10.1196/annals.1316.056. PMID 15542749.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.