Thiethylperazine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 60% |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.381 |
| Chemical and physical data | |
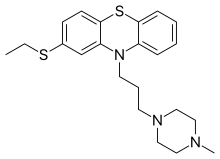
| Formula | C22H29N3S2 |
| Molar mass | 399.62 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Thiethylperazine (Torecan) is an antiemetic of the phenothiazine class. Though it was never licensed or used as an antipsychotic, it may have such effects.
Thiethylperazine activates the transport protein ABCC1 that clears beta-amyloid from brains of mice.[1]
References
- ↑ Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, et al. (October 2011). "Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice". The Journal of Clinical Investigation. 121 (10): 3924–31. doi:10.1172/JCI57867. PMC 3195473. PMID 21881209. Lay summary – Science Daily.
{{cite journal}}: Cite uses deprecated parameter|lay-url=(help)
| Benzimidazoles (*) | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.