CY-208,243
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
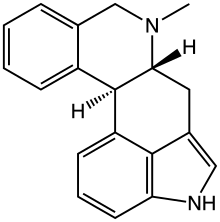
| Formula | C19H18N2 |
| Molar mass | 274.367 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
CY-208,243 is a drug which acts as a dopamine agonist selective for the D1 subtype. Unlike most D1-selective agonists, it shows efficacy in animal models of Parkinson's disease.[1][2][3][4][5]
References
- ↑ Gagnon C, Gomez-Mancilla B, Markstein R, Bédard PJ, Di Paolo T (July 1995). "Effect of adding the D-1 agonist CY 208-243 to chronic bromocriptine treatment of MPTP-monkeys: regional changes of brain dopamine receptors". Progress in Neuro-psychopharmacology & Biological Psychiatry. 19 (4): 667–76. doi:10.1016/0278-5846(95)00110-h. PMID 8588064.
- ↑ Beninger RJ, Rolfe NG (December 1995). "Dopamine D1-like receptor agonists impair responding for conditioned reward in rats". Behavioural Pharmacology. 6 (8): 785–793. doi:10.1097/00008877-199512000-00003. PMID 11224381.
- ↑ Luquin MR, Guillén J, Legarda I, Cruz Rodriguez M, Del Rio L, Dominguez J, Martínez-Lage JM (1996). "Pulsatile administration of D1 but not D2 dopamine agonists induces behavioral tolerance in MPTP-treated monkeys". Advances in Neurology. 69: 239–44. PMID 8615134.
- ↑ D'Aquila PS, Panin F, Cossu M, Peana AT, Serra G (January 2003). "Dopamine D1 receptor agonists induce penile erections in rats". European Journal of Pharmacology. 460 (1): 71–4. doi:10.1016/S0014-2999(02)02881-9. PMID 12535862.
- ↑ Salamone JD, Carlson BB, Rios C, Lentini E, Correa M, Wisniecki A, Betz A (January 2005). "Dopamine agonists suppress cholinomimetic-induced tremulous jaw movements in an animal model of Parkinsonism: tremorolytic effects of pergolide, ropinirole and CY 208-243". Behavioural Brain Research. 156 (2): 173–9. doi:10.1016/j.bbr.2004.05.019. PMID 15582103.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.