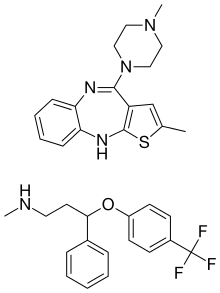
Olanzapine/fluoxetine
 | |
| Combination of | |
|---|---|
| Olanzapine | Atypical antipsychotic |
| Fluoxetine | Selective serotonin reuptake inhibitor |
| Clinical data | |
| Trade names | Symbyax, Cinol Forte, Olapin Forte, others |
| AHFS/Drugs.com | Professional Drug Facts |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| KEGG |
|
| | |
Olanzapine/fluoxetine (trade name Symbyax, created by Eli Lilly and Company) is a fixed-dose combination medication containing olanzapine (Zyprexa), an atypical antipsychotic, and fluoxetine (Prozac), a selective serotonin reuptake inhibitor (SSRI). Olanzapine/fluoxetine is primarily used to treat the depressive episodes of bipolar I disorder as well as treatment-resistant depression.[1]
Medical uses
Olanzapine/fluoxetine was approved by the U.S. Food and Drug Administration (FDA) to treat the depressive episodes of bipolar I disorder in 2003.[1] In 2009, it was granted approval for the treatment of treatment-resistant depression.[2]
Olanzapine/fluoxetine, or other antidepressant/antipsychotic combinations, are sometimes prescribed off-label for anxiety disorders,[3] eating disorders,[4] obsessive–compulsive disorder (OCD),[5] and posttraumatic stress disorder (PTSD).[6]
Side effects
Possible side effects of olanzapine/fluoxetine include all those of the two component drugs: olanzapine (side effects) and fluoxetine (side effects). Common side effects include suicidal thoughts, increased appetite, weight gain, drowsiness, fatigue, dry mouth, swelling, tremor, blurred vision, and difficulty concentrating.[1]
Olanzapine/fluoxetine could produce a severe allergic reaction and should not be used if the patient has previously experienced an allergic reaction to either fluoxetine or olanzapine.[7]
Olanzapine is correlated with an increase in blood sugar. Patients with diabetes, or those at risk for developing it, require careful monitoring.[7]
In rare cases, olanzapine/fluoxetine may cause neuroleptic malignant syndrome.[1]
Like other SSRIs, olanzapine/fluoxetine carries a boxed warning stating that it could increase the risk of suicidal thoughts and behaviors in patients aged 24 and under. The warning also states that olanzapine/fluoxetine may increase the risk of death in elderly patients with dementia-related psychosis.[1]
See also
References
- 1 2 3 4 5 6 "Symbyax- olanzapine and fluoxetine hydrochloride capsule". DailyMed. 21 April 2020. Retrieved 30 September 2020.
- ↑ Grohol, J. "FDA Approves Symbyax for Treatment Resistant Depression". Psych Central Blog.
- ↑ McIntyre R, Katzman M (2003). "The role of atypical antipsychotics in bipolar depression and anxiety disorders". Bipolar Disorders. 5 Suppl 2: 20–35. doi:10.1111/j.1399-2406.2003.00061.x. PMID 14700010.
- ↑ Pederson KJ, Roerig JL, Mitchell JE (2003). "Towards the pharmacotherapy of eating disorders". Expert Opin. Pharmacother. 4 (10): 1659–78. doi:10.1517/14656566.4.10.1659. PMID 14521477. S2CID 38506292.
- ↑ Koran LM, Ringold AL, Elliott MA (2000). "Olanzapine augmentation for treatment-resistant obsessive-compulsive disorder". J Clin Psychiatry. 61 (7): 514–7. doi:10.4088/JCP.v61n0709. PMID 10937610.
- ↑ Stein MB, Kline NA, Matloff JL (2003). "Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study". Am J Psychiatry. 159 (10): 1777–9. doi:10.1176/appi.ajp.159.10.1777. PMID 12359687.
- 1 2 Drugs.com https://www.drugs.com/pdr/symbyax.html
External links
- "Fluoxetine hydrochloride mixture with Olanzapine". Drug Information Portal. U.S. National Library of Medicine.
| Anticonvulsants | |
|---|---|
| Atypical antipsychotics | |
| Others |
|
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||