Frovatriptan
 | |
| Names | |
|---|---|
| Trade names | Frova, Migard, others |
| Other names | Frovatriptan succinate, 6-methylamino-6,7,8,9-tetrahydro-5H-carbazole-3-carboxamide (6R)-6-methylamino-6,7,8,9-tetrahydro-5H-carbazole-3-carboxamide |
IUPAC name
| |
| Clinical data | |
| Drug class | Triptan[1] |
| Main uses | Migraine headaches[1] |
| Side effects | Tiredness, numberness, flushing, dry mouth, pain, heart burn, trouble sleeping[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 2.5 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604013 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 20–30% |
| Metabolism | Liver |
| Elimination half-life | 26 hours |
| Excretion | Kidney |
| Chemical and physical data | |
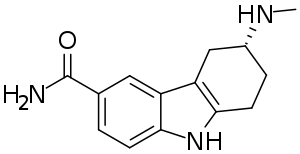
| Formula | C14H17N3O |
| Molar mass | 243.310 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Frovatriptan, sold under the brand name Frova among others, is a medication used to treat migraine headaches.[1] It may also be used to prevent menstrual migraine.[2] It is taken by mouth.[2]
Common side effects include tiredness, numberness, flushing, dry mouth, pain, heart burn, and trouble sleeping.[1] Other side effects may include serotonin syndrome, high blood pressure, heart attack, and medication overuse headaches.[1] Safety in pregnancy is unclear, and use within 24 hours of breastfeeding is not recommended.[3] It is a triptan.[1]
was approved for medical use in the United States in 2001.[1] It is available as a generic medication. In the United Kingdom 6 pills of 2.5 mg costs the NHS about £6 as of 2021.[2] This amount in the United States costs about 67 USD.[4]
Medical uses
Frovatriptan is used in the treatment of migraine.[5] It may also be used for short term prevention of menstrual migraine.[6]
Dosage
It is available as 2.5 mg tablets. One is recommended as soon as the headache starts, with a maximum dose of 5 mg per day.[2]
Contraindications
Frovatriptan should not be given to patients with:
- Ischemic heart disease
- Cerebrovascular syndrome
- Peripheral vascular disease
- Uncontrolled hypertension
- Hemiplegic or basilar migraine
Side effects
Rare, but serious cardiac events have been reported in patients with risk factors predictive of CAD. These include: coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia and ventricular fibrillation.
Pharmacology
Pharmacodynamics
Frovatriptan is a serotonin receptor agonist, with high affinity for the 5-HT1B/1D receptors. It has no significant effects on the GABAA mediated channel activity and benzodiazepine binding sites. Frovatriptan inhibits excessive dilation of arteries that supply blood to the head.
Pharmacokinetics
Frovatriptan has mean terminal elimination half-life of approximately 26 hours, which is substantially longer than other triptans.
Society and culture
The product is licensed to Endo Pharmaceuticals in North America and Menarini in Europe.[7]
Licensing
Frovatriptan is available only by prescription in the United States and Canada, where a secondary New Drug Approval (sNDA) was filed in July 2006.[8]
References
- 1 2 3 4 5 6 7 8 "Frovatriptan Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 12 December 2021.
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 500. ISBN 978-0857114105.
- ↑ "Frovatriptan (Frova) Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 12 December 2021.
- ↑ "Frovatriptan Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 11 October 2016. Retrieved 12 December 2021.
- ↑ Allais G, Benedetto C (2016). "Spotlight on frovatriptan: a review of its efficacy in the treatment of migraine". Drug Design, Development and Therapy. 10: 3225–3236. doi:10.2147/DDDT.S105932. PMC 5055118. PMID 27757013.
- ↑ MacGregor EA (2014). "A review of frovatriptan for the treatment of menstrual migraine". International Journal of Women's Health. 6: 523–35. doi:10.2147/IJWH.S63444. PMC 4039425. PMID 24904224.
- ↑ "Frova". Vernalis. Archived from the original on 2007-09-27. Retrieved 2007-11-28.
- ↑ "Patient Information Sheet -- Frovatriptan succinate (marketed as Frova)". Food and Drug Administration. July 2006. Archived from the original on 2007-09-29. Retrieved 2007-11-28.
External links
| Identifiers: |
|---|