Batanopride
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
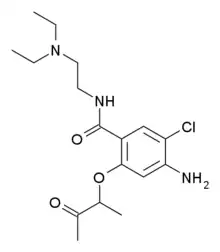
| Formula | C17H26ClN3O3 |
| Molar mass | 355.86 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Batanopride (BMY-25,801) is an antiemetic drug of the benzamide class which acts as a selective 5-HT3 receptor antagonist.[1] It was trialled to reduce nausea during cancer chemotherapy, but was never approved for medical use due to dose-limiting side effects including hypotension and long QT syndrome.[2]
References
- ↑ Gylys JA, Wright RN, Nicolosi WD, Buyniski JP, Crenshaw RR (March 1988). "BMY-25801, an antiemetic agent free of D2-dopamine receptor antagonist properties". The Journal of Pharmacology and Experimental Therapeutics. 244 (3): 830–7. PMID 2978041.
- ↑ Fleming GF, Vokes EE, McEvilly JM, Janisch L, Francher D, Smaldone L (1991). "Double-blind, randomized crossover study of metoclopramide and batanopride for prevention of cisplatin-induced emesis". Cancer Chemotherapy and Pharmacology. 28 (3): 226–7. doi:10.1007/bf00685516. PMID 1855280. S2CID 22520773.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.