WAY-161503
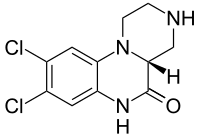 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C11H11Cl2N3O |
| Molar mass | 272.13 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
WAY-161503 is a full agonist of 5-HT2C receptors (Ki = 3.3 nM for displacement of DOI), ~6-fold less potent at 5-HT2A receptors (Ki = 18 nM) and 20-fold less potent at 5-HT2B receptors (Ki = 60 nM).[1] In functional studies, it stimulates calcium mobilization coupled to 5-HT2C, 5-HT2B, and 5-HT2A receptors with EC50 values of 0.8, 1.8, and 7 nM, respectively.[1] WAY-161503 has been reported to produce dose-dependent decreases in food intake in 24-hour fasted normal Sprague-Dawley rats, diet-induced obese mice, and obese Zucker rats with ED50 values of 1.9, 6.8, and 0.73 mg/kg, respectively.[1]
WAY-161503 has been used to examine the role of 5-HT2C receptors in rodent models of depression, locomotion, reinforcement, or motivated behaviors.[2][3]
References
- 1 2 3 Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, et al. (February 2006). "Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503". Brain Research. 1073–1074: 240–51. doi:10.1016/j.brainres.2005.12.052. PMID 16430874. S2CID 23160473.
- ↑ Cryan JF, Lucki I (December 2000). "Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors". The Journal of Pharmacology and Experimental Therapeutics. 295 (3): 1120–6. PMID 11082448.
- ↑ Hayes DJ, Mosher TM, Greenshaw AJ (February 2009). "Differential effects of 5-HT2C receptor activation by WAY 161503 on nicotine-induced place conditioning and locomotor activity in rats". Behavioural Brain Research. 197 (2): 323–30. doi:10.1016/j.bbr.2008.08.034. PMID 18805442. S2CID 16911749.