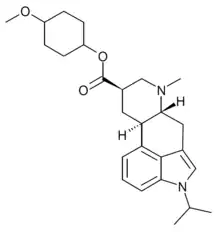
Sergolexole
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H36N2O3 |
| Molar mass | 424.585 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sergolexole (developmental code name LY-281,067) is an ergoline derivative which acts as a selective antagonist of the serotonin 5-HT2 receptors. It has been used for various research applications, but was never developed for medical use.[1][2][3]
References
- ↑ Cohen ML, Fuller RW, Kurz KD, Parli CJ, Mason NR, Meyers DB, et al. (January 1988). "Preclinical pharmacology of a new serotonergic receptor antagonist, LY281067". The Journal of Pharmacology and Experimental Therapeutics. 244 (1): 106–12. PMID 3335993.
- ↑ Cohen ML, Parli CJ, Fuller RW (December 1989). "5-Hydroxytryptamine2 receptor antagonist activity of the acid metabolite (1-isopropyl dihydrolysergic acid) of the ergoline ester, sergolexole (LY281067)". The Journal of Pharmacology and Experimental Therapeutics. 251 (3): 1006–11. PMID 2600800.
- ↑ Koba S, Pakala R, Watanabe T, Katagiri T, Benedict CR (November 1999). "Vascular smooth muscle proliferation: synergistic interaction between serotonin and low density lipoproteins". Journal of the American College of Cardiology. 34 (5): 1644–51. doi:10.1016/s0735-1097(99)00349-6. PMID 10551718.
| Lysergic acid derivatives |
|
|---|---|
| Psychedelic lysergamides |
|
| Clavines |
|
| Other ergolines |
|
| Natural sources |
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui) |
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.