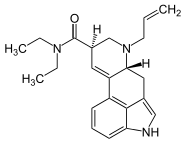
AL-LAD
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H27N3O |
| Molar mass | 349.478 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
AL-LAD, also known as 6-allyl-6-nor-LSD, is a psychedelic drug and an analog of lysergic acid diethylamide (LSD).[2] It is described by Alexander Shulgin in the book TiHKAL (Tryptamines i Have Known And Loved). It is synthesized starting from nor-LSD as a precursor, using allyl bromide as a reactant.
Effects in humans

While AL-LAD has subtly different effects than LSD, and appears to be slightly shorter lasting, their potencies are similar;[3][4] an active dose of AL-LAD is reported to be between 50 and 150 micrograms.[5] AL-LAD has a known but short and highly uncommon history of recreational human use, which originated in Ireland and the UK, but spread internationally.
Chemistry
AL-LAD does not cause a color change with the Marquis, Mecke or Mandelin reagents,[6] but does cause the Ehrlich's reagent to turn purple because of the presence of the indole moiety in its structure.
Legal status
AL-LAD is not scheduled by the United Nations' Convention on Psychotropic Substances.[7]
Denmark
AL-LAD is illegal in Denmark.[8]
Latvia
AL-LAD is possibly illegal in Latvia. Although it isn't specifically scheduled, it may be controlled as an LSD structural analog due to an amendment made on June 1, 2015.[9]
Romania
AL-LAD is illegal in Romania. It is not included directly in the list of controlled substances, but it is included in an analogue act
Sweden
The Riksdag added AL-LAD to Narcotic Drugs Punishments Act under swedish schedule I ("substances, plant materials and fungi which normally do not have medical use" ) as of January 26, 2016, published by Medical Products Agency (MPA) in regulation HSLF-FS 2015:35 listed as 6-allyl-6-nor-LSD, AL-LAD, and 6-allyl-N,N-dietyl-9,10-didehydroergolin-8-karboxamid.[10]
Switzerland
AL-LAD is illegal in Switzerland.[11]
United Kingdom
AL-LAD is illegal in the UK. On June 10, 2014 the UK Advisory Council on the Misuse of Drugs (ACMD) recommended that AL-LAD be specifically named in the UK Misuse of Drugs Act as a class A drug despite not identifying any harm associated with its use.[12] The UK Home office accepted this advice and announced a ban of the substance to be enacted on 6 January 2015 as part of The Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014.
United States
AL-LAD is not scheduled as a controlled substance at the federal level in the United States,[13] but AL-LAD could legally be considered an analog of LSD, in which case, sales or possession with intent for human consumption could be prosecuted under the Federal Analogue Act.[14]
See also
References
- ↑ "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- ↑ Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, et al. (January 2017). "6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2'S,4'S)-lysergic acid 2,4-dimethylazetidide (LSZ)". Drug Testing and Analysis. 9 (1): 38–50. doi:10.1002/dta.1985. PMC 5411264. PMID 27265891.
- ↑ Schifano F, Orsolini L, Papanti D, Corkery J (June 2016). "NPS: Medical Consequences Associated with Their Intake". Current Topics in Behavioral Neurosciences. Current Topics in Behavioral Neurosciences. Vol. 32. Springer International Publishing. pp. 351–380. doi:10.1007/7854_2016_15. ISBN 978-3-319-52442-9. OCLC 643052237. PMID 27272067.
- ↑ Hoffman AJ, Nichols DE (September 1985). "Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives". Journal of Medicinal Chemistry. 28 (9): 1252–5. doi:10.1021/jm00147a022. PMID 4032428.
- ↑ Shulgin, Alexander (1997). TiHKAL: The Continuation. Berkeley, California: Transform Press. p. 392. ISBN 978-0-9630096-9-2. Archived from the original on 2015-11-18.
- ↑ Ecstasydata. "EcstasyData.org - AL-LAD (Not sold as ecstasy)". Archived from the original on 2013-12-26. Retrieved 2013-12-25.
- ↑ simone.rupprich. "Conventions". www.unodc.org. Archived from the original on 12 January 2018. Retrieved 4 May 2018.
- ↑ "Lists of euphoriant substances". The Danish Medicines Agency. September 2015. Archived from the original on 2016-06-09.
- ↑ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem". LIKUMI.LV. Archived from the original on 4 May 2018. Retrieved 4 May 2018.
- ↑ "Gemensamma författningssamlingen avseende hälso- och sjukvård, socialtjänst, läkemedel, folkhälsa m.m." [Joint constitutional collection on health care, social services, pharmaceuticals, public health, etc.] (PDF). Lakemedelsverket (in Swedish). Archived (PDF) from the original on 2017-10-31. Retrieved 2017-04-21.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Der Bundesrat. Archived from the original on 2016-01-23.
- ↑ ACMD (10 June 2014). "Update of the Generic Definition for Tryptamines" (PDF). UK Home Office. p. 12. Archived (PDF) from the original on 6 October 2014. Retrieved 10 June 2014.
- ↑ "PART 1308 - Section 1308.11 Schedule I". www.deadiversion.usdoj.gov. Archived from the original on 27 August 2009. Retrieved 4 May 2018.
- ↑ "Erowid Analog Law Vault : Federal Controlled Substance Analogue Act Summary". www.erowid.org. Archived from the original on 17 April 2018. Retrieved 4 May 2018.
Further reading
- Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB (April 1995). "LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors". Psychopharmacology. 118 (4): 401–9. doi:10.1007/BF02245940. PMID 7568626. S2CID 21484356.
- Niwaguchi T, Nakahara Y, Ishii H (May 1976). "[Studies on lysergic acid diethylamide and related compounds. IV. Syntheses of various amide derivatives of norlysergic acid and related compounds]". Yakugaku Zasshi. 96 (5): 673–8. doi:10.1248/yakushi1947.96.5_673. PMID 987200.
- >Pfaff RC, Huang X, Marona-Lewicka D, Oberlender R, Nichols DE (1994). "Lysergamides revisited" (PDF). Hallucinogens: An Update. NIDA Research Monograph. Vol. 146. United States Department of Health and Human Services. pp. 52–73. PMID 8742794. Archived from the original (PDF) on 2015-07-23.