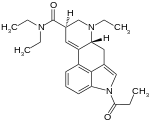
1P-ETH-LAD
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H31N3O2 |
| Molar mass | 393.531 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
1P-ETH-LAD (1-propionyl-6-ethyl-6-nor-lysergic acid diethyamide) is an analog of LSD. 1P-ETH-LAD is a psychedelic drug similar to LSD. Research has shown formation of ETH-LAD from 1P-ETH-LAD incubated in human serum, suggesting that it functions as a prodrug.[2] It is part of the lysergamide chemical class. Like ETH-LAD, this drug has been reported to be significantly more potent than LSD itself, and is reported to largely mimic ETH-LAD's psychedelic effects.
1P-ETH-LAD has little history of human usage before January 2016.
Legal issues
- United States: 1P-ETH-LAD may be considered illegal in the U.S. for human consumption under the Federal Analogue Act.
- United Kingdom: It is illegal to produce, supply, or import this substance under the Psychoactive Substance Act, which came into effect on May 26th, 2016.[3]
See also
References
- ↑ "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- ↑ Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Stratford A, et al. (October 2017). "6 -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD)". Drug Testing and Analysis. 9 (10): 1641–1649. doi:10.1002/dta.2196. PMC 6230477. PMID 28342178.
- ↑ "Psychoactive Substances Act 2016". Legislation.gov.uk.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.