2C-CP
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| Chemical and physical data | |
| Formula | C13H19NO2 |
| Molar mass | 221.300 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
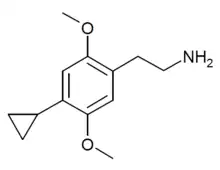
2C-CP (2C-cP) is a recreational designer drug from the substituted phenethylamine family, with psychedelic effects. It was first synthesised by Daniel Trachsel and colleagues in 2006. It has a binding affinity (Ki) of 95 nM at the serotonin receptor 5-HT2A and 41 nM at 5-HT2C and is active at a dosage of between 15 and 35 mg with a duration of 3 to 6 hours.[1]
See also
References
- ↑ Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine Von der Struktur zur Funktion [Phenethylamine From structure to function] (in German). Nachtschatten Verlag AG. p. 766-771. ISBN 978-3-03788-700-4.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.