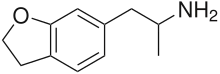
6-APDB
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO |
| Molar mass | 177.247 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
6-(2-Aminopropyl)-2,3-dihydrobenzofuran (6-APDB, 4-Desoxy-MDA, EMA-3) is a stimulant and entactogen drug of the phenethylamine and amphetamine classes.[1] It is an analogue of MDA where the heterocyclic 4-position oxygen from the 3,4-methylenedioxy ring has been replaced with a methylene bridge.[1] 5-APDB (3-Desoxy-MDA) is an analogue of 6-APDB where the 3-position oxygen has been replaced with a methylene instead.[1] 6-APDB, along with 5-APDB, was first synthesized by David E. Nichols in the early 1990s while investigating non-neurotoxic MDMA analogues.[1]
In animal studies, 6-APDB fully substitutes for MBDB and MMAI but not for amphetamine or LSD.[1] In vitro, 6-APDB has been shown to inhibit the reuptake of serotonin, dopamine, and norepinephrine with IC50 values of 322 nM, 1,997 nM, and 980 nM, respectively.[1] These values are very similar to those of MDA, but with those for the catecholamines slightly lower in comparison, perhaps more similarly to MDMA.[1] In contrast, 5-APDB is highly selective for serotonin.[1] Though 6-APDB does not substitute for amphetamine in rats at the doses used in referenced study, based on its in vitro profile it can be suggested that it may have amphetamine-like effects at higher doses.
The unsaturated benzofuran derivative 6-APB, or 6-(2-aminopropyl)benzofuran is also known, but the difference in pharmacological effects between 6-APB and 6-APDB is unclear.
6-APDB is a class B drug in the UK since June 10, 2013. It is banned by a blanket law on benzofurans and related compounds.[2]
References
- 1 2 3 4 5 6 7 8 Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE (November 1993). "Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine". Journal of Medicinal Chemistry. 36 (23): 3700–6. doi:10.1021/jm00075a027. PMID 8246240.
- ↑ UK Home Office (2014-03-05). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government. Retrieved 2014-03-11.