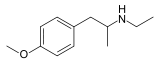
Para-Methoxy-N-ethylamphetamine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.839 |
| Chemical and physical data | |
| Formula | C12H19NO |
| Molar mass | 193.290 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
para-Methoxyethylamphetamine (PMEA), is a stimulant drug related to PMA. PMEA reputedly produces similar effects to PMA, but is considerably less potent[1] and seems to have slightly less tendency to produce severe hyperthermia, at least at low doses. At higher doses however the side effects and danger of death approach those of PMA itself, and PMEA should still be considered a potentially dangerous drug. Investigation of a drug-related death in Japan in 2005 showed PMEA to be present in the body and was thought to be responsible for the death.[2]
See also
- 3-Fluoroethamphetamine
- 3,4-Methylenedioxyethamphetamine
- Fenfluramine
References
- ↑ Bustamante D, Díaz-Véliz G, Paeile C, Zapata-Torres G, Cassels BK (October 2004). "Analgesic and behavioral effects of amphetamine enantiomers, p-methoxyamphetamine and n-alkyl-p-methoxyamphetamine derivatives". Pharmacology, Biochemistry, and Behavior. 79 (2): 199–212. doi:10.1016/j.pbb.2004.06.017. PMID 15501295.
- ↑ Zaitsu K, Katagi M, Kamata T, Kamata H, Shima N, Tsuchihashi H, et al. (May 2008). "Determination of a newly encountered designer drug "p-methoxyethylamphetamine" and its metabolites in human urine and blood". Forensic Science International. 177 (1): 77–84. doi:10.1016/j.forsciint.2007.11.001. PMID 18155375.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.