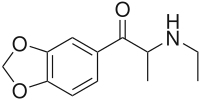
Ethylone
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, nasal, IV |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Ethylone, also known as 3,4-methylenedioxy-N-ethylcathinone (MDEC, βk-MDEA), is a recreational designer drug classified as an entactogen, stimulant, and psychedelic of the phenethylamine, amphetamine, and cathinone chemical classes. It is the β-keto analogue of MDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone. In the United States, it began to be found in cathinone products in late 2011.[1]
Very little data exists about the pharmacological properties, metabolism, and toxicity of ethylone, and although several ethylone-related deaths have been reported, the cause of death was not due to ingestion of ethylone.[1]
Pharmacokinetics
Analysis of human and rat urine for the metabolites of bk-amphetamines suggested that ethylone was degraded in the following metabolic steps:[2]
- N-deethylation to the primary amine.
- Reduction of the keto moiety to the respective alcohol.
Legal Status
As of October 2015 Ethylone is a controlled substance in China.[3]
See also
- 5-Methylethylone
- Benzylone
References
- 1 2 Lee D, Chronister CW, Hoyer J, Goldberger BA (September 2015). "Ethylone-Related Deaths: Toxicological Findings". Journal of Analytical Toxicology. 39 (7): 567–71. doi:10.1093/jat/bkv053. PMID 26025164.
- ↑ Meyer MR, Wilhelm J, Peters FT, Maurer HH (June 2010). "Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry". Analytical and Bioanalytical Chemistry. 397 (3): 1225–33. doi:10.1007/s00216-010-3636-5. PMID 20333362. S2CID 21471611.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
External links
Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| Adamantanes | |
|---|---|
| Adenosine antagonists |
|
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics |
|
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines |
|
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines |
|
| Others |
|
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|