Dianicline
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
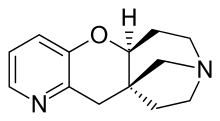
| Formula | C13H16N2O |
| Molar mass | 216.278 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Dianicline (SSR-591,813) is a drug developed by Sanofi-Aventis which acts as a partial agonist at neural nicotinic acetylcholine receptors. It is subtype-selective, binding primarily to the α4β2 subtype. It is being developed as a medication for the treatment of nicotine dependence to assist in smoking cessation.[1] Dianicline is very similar to the already marketed drug varenicline and it is unclear what advantages it will have over the older drug, although it may have an improved side effect profile. It has been through human trials up to Phase II, although results have not yet been reported. Drug development has been discontinued after reporting of unfavourable results during Phase III trials.[2][3][4]
References
- ↑ Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, et al. (July 2003). "SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation". The Journal of Pharmacology and Experimental Therapeutics. 306 (1): 407–20. doi:10.1124/jpet.103.049262. PMID 12682217. S2CID 37543790.
- ↑ Fagerström K, Balfour DJK. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opinion on Investigational Drugs. 2006; 15(2):107-116.
- ↑ Efficacy and Safety of Dianicline Versus Placebo as an Aid to Smoking Cessation
- ↑ "Sanofi Pipeline" (PDF). Archived from the original (PDF) on 2008-12-04. Retrieved 2009-06-24.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.