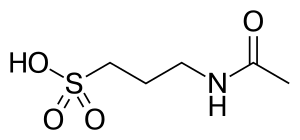
Acamprosate
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /əˈkæmproʊseɪt/ |
| Trade names | Campral, Aotal, others[1] |
| Other names | N-Acetyl homotaurine, Acamprosate calcium (JAN JP), Acamprosate calcium (USAN US) |
IUPAC name
| |
| Clinical data | |
| Main uses | Alcohol dependence[1] |
| Side effects | Diarrhea, weakness[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 11%[3] |
| Protein binding | Negligible[3] |
| Metabolism | Nil[3] |
| Elimination half-life | 20 h to 33 h[3] |
| Excretion | Kidney[3] |
| Chemical and physical data | |
| Formula | C5H11NO4S |
| Molar mass | 181.21 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Acamprosate, sold under the brand name Campral among others, is a medication used along with counselling to treat alcohol dependence.[1] When used alone, it is not effective for most individuals;[4] it works best when used in combination with counselling.[1][5] It is taken by mouth.[6]
Common side effects include diarrhea and weakness.[2] Serious side effects may include allergic reactions, mood changes, thoughts of suicide, and heart palpitations.[7] A lower dose is recommended in people with mild kidney problems and use is not recommended in people with severe kidney disease.[2] Use is okay in mild to moderate liver dysfunction.[8] Safety during pregnancy is unclear.[9] Acamprosate is thought to work by altering chemical signaling in the brain.[8]
Acamprosate was approved for medical use in the United States in 2004.[2] It is on the World Health Organization's List of Essential Medicines.[10] It is available as a generic medication in the United Kingdom were a month of treatment costs the NHS about 30 pounds as of 2020.[6] This amount in the United States costs as little as 80 USD as of 2020.[11]
Medical uses
Acamprosate is useful when used along with counseling in the treatment of alcohol dependence.[1] It should be started once a person has managed to stop drinking.[2] Over three to twelve months it increases the number of people who do not drink at all by about 1 in 12 and the number of days without alcohol.[1][12]
Acamprosate can be continued even if the person returns to drinking alcohol.[2] The other medications naltrexone and disulfiram may also be used by those taking acamprosate.[2] It appears to work as well as naltrexone.[1]
Dosage
In adults who weight less than 60 kg the typical dose is 666 mg before breakfast and 333 mg at lunch and before bed.[6] In those who weight more than 60 kg the dose is 666 mg three times per day.[6]
Side effects
The US label carries warnings about increases of suicidal behavior, major depressive disorder, and kidney failure.[3]
Side effects that caused people to stop taking the drug in clinical trials included diarrhea, nausea, depression, and anxiety.[3]
Potential side effects include headache, stomach pain, back pain, muscle pain, joint pain, chest pain, infections, flu-like symptoms, chills, heart palpitations, high blood pressure, fainting, vomiting, upset stomach, constipation, increased appetite, weight gain, edema, sleepiness, decreased sex drive, impotence, forgetfulness, abnormal thinking, abnormal vision, distorted sense of taste, tremors, runny nose, coughing, difficulty breathing, sore throat, bronchitis, and rashes.[3]
Contraindications
Acamprosate is primarily removed by the kidneys and should not be given to people with severely impaired kidneys (creatinine clearance less than 30 mL/min). A dose reduction is suggested in those with moderately impaired kidneys (creatinine clearance between 30 mL/min and 50 mL/min).[3][13] It is also contraindicated in those who have a strong allergic reaction to acamprosate calcium or any of its components.[13]
Pharmacology
Pharmacodynamics
The pharmacodynamics of acamprosate are complex and not fully understood;[14][15][16] however, it is believed to act as an NMDA receptor antagonist and positive allosteric modulator of GABAA receptors.[15][16]
Its activity on those receptors is indirect, unlike that of most other agents used in this context.[17] An inhibition of the GABA-B system is believed to cause indirect enhancement of GABAA receptors.[17] The effects on the NMDA complex are dose-dependant; the product appears to enhance receptor activation at low concentrations, while inhibiting it when consumed in higher amounts, which counters the excessive activation of NMDA receptors in the context of alcohol withdrawal.[18]
The product also increases the endogenous production of taurine.[18]
Ethanol and benzodiazepines act on the central nervous system by binding to the GABAA receptor, increasing the effects of the inhibitory neurotransmitter GABA (i.e., they act as positive allosteric modulators at these receptors).[15][4] In chronic alcohol abuse, one of the main mechanisms of tolerance is attributed to GABAA receptors becoming downregulated (i.e. these receptors become less sensitive to GABA).[4] When alcohol is no longer consumed, these down-regulated GABAA receptor complexes are so insensitive to GABA that the typical amount of GABA produced has little effect, leading to physical withdrawal symptoms;[4] since GABA normally inhibits neural firing, GABAA receptor desensitization results in unopposed excitatory neurotransmission (i.e., fewer inhibitory postsynaptic potentials occur through GABAA receptors), leading to neuronal over-excitation (i.e., more action potentials in the postsynaptic neuron). One of acamprosate's mechanisms of action is the enhancement of GABA signaling at GABAA receptors via positive allosteric receptor modulation.[15][16] It has been purported to open the chloride ion channel in a novel way as it does not require GABA as a cofactor, making it less liable for dependence than benzodiazepines. Acamprosate has been successfully used to control tinnitus, hyperacusis, ear pain and inner ear pressure during alcohol use due to spasms of the tensor tympani muscle.
In addition, alcohol also inhibits the activity of N-methyl-D-aspartate receptors (NMDARs).[19][20] Chronic alcohol consumption leads to the overproduction (upregulation) of these receptors. Thereafter, sudden alcohol abstinence causes the excessive numbers of NMDARs to be more active than normal and to contribute to the symptoms of delirium tremens and excitotoxic neuronal death.[21] Withdrawal from alcohol induces a surge in release of excitatory neurotransmitters like glutamate, which activates NMDARs.[22] Acamprosate reduces this glutamate surge.[23] The drug also protects cultured cells from excitotoxicity induced by ethanol withdrawal[24] and from glutamate exposure combined with ethanol withdrawal.[25]
The substance also helps re-establish a standard sleep architecture by normalizing stage 3 and REM sleep phases, which is believed to be an important aspect of its pharmacological activity.[18]
Pharmacokinetics
Acamprosate is not metabolized by the human body.[16] Acamprosate's absolute bioavailability from oral administration is approximately 11%,[16] and its bioavailability is decreased when taken with food.[26] Following administration and absorption of acamprosate, it is excreted unchanged (i.e., as acamprosate) via the kidneys.[16]
Its absorption and elimination are very slow, with a Tmax of 6h and an elimination half life of over 30h.[17]
History
Acamprosate was developed by Lipha, a subsidiary of Merck KGaA.[27] and was approved for marketing in Europe in 1989.
In October 2001 Forest Laboratories acquired the rights to market the drug in the US.[27][28]
It was approved by the FDA in July 2004.[29]
The first generic versions of acamprosate were launched in the US in 2013.[30]
As of 2015 acamprosate was in development by Confluence Pharmaceuticals as a potential treatment for fragile X syndrome. The drug was granted orphan status for this use by the FDA in 2013 and by the EMA in 2014.[31]
Society and culture
"Acamprosate" is the INN and BAN for this substance. "Acamprosate calcium" is the USAN and JAN. It is also technically known as N-acetylhomotaurine or as calcium acetylhomotaurinate.
It is sold under the brand name Campral.[3]
Research
In addition to its apparent ability to help patients refrain from drinking, some evidence suggests that acamprosate is neuroprotective (that is, it protects neurons from damage and death caused by the effects of alcohol withdrawal, and possibly other causes of neurotoxicity).[23][32]
References
- 1 2 3 4 5 6 7 Plosker, GL (July 2015). "Acamprosate: A Review of Its Use in Alcohol Dependence". Drugs. 75 (11): 1255–68. doi:10.1007/s40265-015-0423-9. PMID 26084940. S2CID 19119078.
- 1 2 3 4 5 6 7 "Acamprosate Calcium Monograph for Professionals". Drugs.com. Archived from the original on 4 November 2019. Retrieved 7 October 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Campral label" (PDF). FDA. January 2012. Archived (PDF) from the original on 27 February 2017. Retrieved 27 November 2017. For label updates see FDA index page for NDA 021431 Archived 2021-08-27 at the Wayback Machine
- 1 2 3 4 Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 16: Reinforcement and Addictive Disorders". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. ISBN 9780071827706.
Unfortunately, acamprosate is not adequately effective for most alcoholics.
- ↑ Nutt, DJ (2014). "Doing it by numbers: A simple approach to reducing the harms of alcohol". Journal of Psychopharmacology. 28 (1): 3–7. doi:10.1177/0269881113512038. PMID 24399337. S2CID 36860967. Archived from the original on 2021-08-27. Retrieved 2020-01-22.
- 1 2 3 4 BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 509. ISBN 9780857113658.
- ↑ "Acamprosate". drugs.com. 2005-03-25. Archived from the original on 22 December 2006. Retrieved 2007-01-08.
- 1 2 Williams, SH. (2005). "Medications for treating alcohol dependence". American Family Physician. 72 (9): 1775–1780. PMID 16300039. Archived from the original on 2007-09-29. Retrieved 2006-11-29.
- ↑ "Acamprosate (Campral) Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 7 October 2020.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Acamprosate Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 26 September 2016. Retrieved 7 October 2020.
- ↑ Ton, Joey (18 February 2020). "#253 Pharmacologic management of alcohol use disorder: worth a shot?". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 15 June 2023.
- 1 2 Saivin, S; Hulot, T; Chabac, S; Potgieter, A; Durbin, P; Houin, G (Nov 1998). "Clinical Pharmacokinetics of Acamprosate". Clinical Pharmacokinetics. 35 (5): 331–345. doi:10.2165/00003088-199835050-00001. PMID 9839087. S2CID 34047050.
- ↑ "Acamprosate: Biological activity". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Archived from the original on 1 December 2017. Retrieved 26 November 2017.
Due to the complex nature of this drug's MMOA, and a paucity of well defined target affinity data, we do not map to a primary drug target in this instance.
- 1 2 3 4 "Acamprosate: Summary". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Archived from the original on 1 December 2017. Retrieved 26 November 2017.
Acamprosate is a NMDA glutamate receptor antagonist and a positive allosteric modulator of GABAA receptors.
Marketed formulations contain acamprosate calcium - 1 2 3 4 5 6 "Acamprosate". DrugBank. University of Alberta. 19 November 2017. Archived from the original on 1 December 2017. Retrieved 26 November 2017.
Acamprosate is thought to stabilize the chemical balance in the brain that would otherwise be disrupted by alcoholism, possibly by blocking glutaminergic N-methyl-D-aspartate receptors, while gamma-aminobutyric acid type A receptors are activated. ... The mechanism of action of acamprosate in maintenance of alcohol abstinence is not completely understood. Chronic alcohol exposure is hypothesized to alter the normal balance between neuronal excitation and inhibition. in vitro and in vivo studies in animals have provided evidence to suggest acamprosate may interact with glutamate and GABA neurotransmitter systems centrally, and has led to the hypothesis that acamprosate restores this balance. It seems to inhibit NMDA receptors while activating GABA receptors.
- 1 2 3 Kalk, Nicola J; Lingford-Hughes, Anne R (February 2014). "The clinical pharmacology of acamprosate". British Journal of Clinical Pharmacology. 77 (2): 315–323. doi:10.1111/bcp.12070. ISSN 0306-5251. PMC 4014018. PMID 23278595. Archived from the original on 2021-04-14. Retrieved 2020-10-06.
- 1 2 3 Mason, Barbara J.; Heyser, Charles J. (March 2010). "Acamprosate: A prototypic neuromodulator in the treatment of alcohol dependence". CNS & neurological disorders drug targets. 9 (1): 23–32. ISSN 1871-5273. PMC 2853976. PMID 20201812. Archived from the original on 2020-11-11. Retrieved 2020-10-06.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 372. ISBN 9780071481274.
- ↑ Möykkynen T, Korpi ER (July 2012). "Acute effects of ethanol on glutamate receptors". Basic & Clinical Pharmacology & Toxicology. 111 (1): 4–13. doi:10.1111/j.1742-7843.2012.00879.x. PMID 22429661.
- ↑ Tsai, G; Coyle, JT (1998). "The role of glutamatergic neurotransmission in the pathophysiology of alcoholism". Annual Review of Medicine. 49: 173–84. doi:10.1146/annurev.med.49.1.173. PMID 9509257.
- ↑ Tsai, GE; Ragan, P; Chang, R; Chen, S; Linnoila, VM; Coyle, JT (1998). "Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal". The American Journal of Psychiatry. 155 (6): 726–32. doi:10.1176/ajp.155.6.726 (inactive 2020-08-24). PMID 9619143. Archived from the original on 2011-09-27. Retrieved 2007-03-04.
{{cite journal}}: CS1 maint: DOI inactive as of August 2020 (link) - 1 2 De Witte, P; Littleton, J; Parot, P; Koob, G (2005). "Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action". CNS Drugs. 19 (6): 517–37. doi:10.2165/00023210-200519060-00004. PMID 15963001. S2CID 11563216.
- ↑ Mayer, S; Harris, BR; Gibson, DA; Blanchard, JA; Prendergast, MA; Holley, RC; Littleton, J (2002). "Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus". Alcoholism: Clinical and Experimental Research. 26 (10): 1468–78. doi:10.1097/00000374-200210000-00003. PMID 12394279.
- ↑ Al Qatari, M; Khan, S; Harris, B; Littleton, J (2001). "Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain". Alcoholism: Clinical and Experimental Research. 25 (9): 1276–83. doi:10.1111/j.1530-0277.2001.tb02348.x. PMID 11584146.
- ↑ Trevor, Anthony J. (2017). "The Alcohols". In Katzung, Bertram G. (ed.). Basic & Clinical Pharmacology (14th ed.). New York. ISBN 9781259641152. OCLC 1015240036.
- 1 2 Berfield, Susan (27 May 2002). "A CEO and His Son". Bloomberg Businessweek. Archived from the original on 2017-12-01. Retrieved 2017-11-27.
- ↑ "Press release: Forest Laboratories Announces Agreement For Alcohol Addiction Treatment". Forest Labs via Evaluate Group. October 23, 2001. Archived from the original on August 27, 2021. Retrieved November 27, 2017.
- ↑ "FDA Approves New Drug for Treatment of Alcoholism". FDA Talk Paper. Food and Drug Administration. 2004-07-29. Archived from the original on 2008-01-17. Retrieved 2009-08-15.
- ↑ "Acamprosate generics". DrugPatentWatch. Archived from the original on 1 December 2017. Retrieved 27 November 2017.
- ↑ "Acamprosate - Confluence Pharmaceuticals - AdisInsight". AdisInsight. Archived from the original on 21 May 2021. Retrieved 27 November 2017.
- ↑ Mann K, Kiefer F, Spanagel R, Littleton J (July 2008). "Acamprosate: recent findings and future research directions". Alcohol. Clin. Exp. Res. 32 (7): 1105–10. doi:10.1111/j.1530-0277.2008.00690.x. PMID 18540918.
External links
| Identifiers: |
|---|