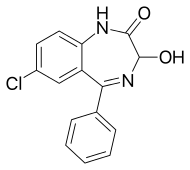
Oxazepam
 | |
 | |
| Names | |
|---|---|
| Trade names | Serax, Alepam, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Benzodiazepine[2] |
| Main uses | Anxiety, trouble sleeping, alcohol withdrawal[3][2] |
| Side effects | Sleepiness, dizziness, headache[2] |
| Interactions | Opioids[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 10 to 30 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 92.8% |
| Metabolism | Liver (glucuronidation) |
| Elimination half-life | 6–9 h[4][5][6] |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H11ClN2O2 |
| Molar mass | 286.71 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 205 to 206 °C (401 to 403 °F) |
SMILES
| |
InChI
| |
Oxazepam is a benzodiazepine used to treat anxiety, trouble sleeping, and alcohol withdrawal.[3][2] It is taken by mouth.[2] It has a relatively slow onset and prolonged effects.[7]
Common side effects include sleepiness, dizziness, and headache.[2] Other side effects may include abuse, agitation, memory loss, suicide, and slurred speech.[3] Use is not recommended during early pregnancy.[2] Use is not generally recommended with opioids.[2] It is believed to work via the GABA neurotransmitter.[2]
Oxazepam was patented in 1962 and approved for medical use in 1964.[8] It is available as a generic medication.[3] In the United Kingdom 28 tablets of 15 mg costs the NHS about £6 as of 2021.[3] In the United States this amount costs about 30 USD.[9]
Medical uses

It is an intermediate-acting benzodiazepine with a slow onset of action,[10] so it is usually prescribed to individuals who have trouble staying asleep, rather than falling asleep. It is commonly prescribed for anxiety disorders with associated tension, irritability, and agitation. It is also prescribed for drug and alcohol withdrawal, and for anxiety associated with depression. Physicians may use oxazepam outside its approved indications to treat social phobia, post-traumatic stress disorder, insomnia, premenstrual syndrome, and other conditions.[11]
Dosage
It is often taken at a dose of 10 to 30 mg once to four times per day.[3]
Contraindications
Oxazepam is contraindicated in myasthenia gravis, chronic obstructive pulmonary disease, and limited pulmonary reserve, as well as severe hepatic disease.
Side effects
The side effects of oxazepam are similar to those of other benzodiazepines, and may include dizziness, drowsiness, headache, memory impairment, paradoxical excitement, and anterograde amnesia, but does not affect transient global amnesia. Side effects due to rapid decrease in dose or abrupt withdrawal from oxazepam may include abdominal and muscle cramps, convulsions, depression, inability to fall asleep or stay asleep, sweating, tremors, or vomiting.[12]
Benzodiazepines require special precautions if used in the elderly, during pregnancy, in children, alcohol- or drug-dependent individuals, and individuals with comorbid psychiatric disorders.[13]
Tolerance and dependence
In September 2020, the U.S. Food and Drug Administration (FDA) required the boxed warning be updated for all benzodiazepines to describe the risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions consistently across all the medicines in the class.[14]
Oxazepam, as with other benzodiazepine drugs, can cause tolerance, physical dependence, addiction, and benzodiazepine withdrawal syndrome. Withdrawal from oxazepam or other benzodiazepines often leads to withdrawal symptoms which are similar to those seen during alcohol and barbiturate withdrawal. The higher the dose and the longer the drug is taken, the greater the risk of experiencing unpleasant withdrawal symptoms. Withdrawal symptoms can occur, though, at standard dosages and also after short-term use. Benzodiazepine treatment should be discontinued as soon as possible by a slow and gradual dose reduction regimen.[15]
Pregnancy
Oxazepam when taken during the third trimester, causes a definite risk to the neonate including a severe benzodiazepine withdrawal syndrome including hypotonia, and reluctance to suck, to apnoeic spells, cyanosis, and impaired metabolic responses to cold stress. Floppy infant syndrome and sedation in the newborn may also occur. Symptoms of floppy infant syndrome and the neonatal benzodiazepine withdrawal syndrome have been reported to persist from hours to months after birth.[16]
Benzodiazepines including oxazepam are lipophilic drugs and rapidly penetrate membranes, so rapidly crosses over into the placenta with significant uptake of the drug. Use of benzodiazepines in late pregnancy, especially high doses, may result in floppy infant syndrome.[17]
Interactions
As oxazepam is an active metabolite of diazepam, an overlap in possible interactions is likely with other drugs or food, with exception of the pharmacokinetic CYP450 interactions (e.g. with cimetidine). Precautions and following the prescription are required when taking oxazepam (or other benzodiazepines) in combinations with antidepressant medication (SSRIs such as fluoxetine, sertraline, and paroxetine, or multiple reuptake inhibitors such as bupropion, duloxetine, or venlafaxine), potent painkillers (opioids, e.g. morphine, oxycodone or methadone). Concurrent use of these medicines (as well as other benzodiazepines) can interact in a way that is difficult to predict. Drinking alcohol when taking oxazepam is not recommended. Concomitant use of oxazepam and alcohol can lead to increased sedation, severe problems with coordination (ataxia), decreased muscle tone, and in severe cases or in predisposed patients, even to life-threatening intoxications with respiratory depression, coma, and collapse. There is a risk of blood circulation collapse, possibly the same condition as blood circulation syncope Archived 2021-09-05 at the Wayback Machine, when oxazepam is used in combination with quetiapine, an antipsychotic.
Overdose
Oxazepam is generally less toxic in overdose than other benzodiazepines.[18] Important factors which affect the severity of a benzodiazepine overdose include the dose ingested, the age of the patient, and health status prior to overdose. Benzodiazepine overdoses can be much more dangerous if a coingestion of other CNS depressants such as opiates or alcohol has occurred. Symptoms of an oxazepam overdose include:[19][20][21]
- Respiratory depression
- Excessive somnolence
- Altered consciousness
- Central nervous system depression
- Occasionally cardiovascular and pulmonary toxicity
- Rarely, deep coma
Pharmacology
Oxazepam is an intermediate-acting benzodiazepine of the 3-hydroxy family; it acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the central nervous system.[22][23] The half-life of oxazepam is between 6 and 9 hours.[24][5][25] It has been shown to suppress cortisol levels.[26] Oxazepam is the most slowly absorbed and has the slowest onset of action of all the common benzodiazepines according to one British study.[27]
Oxazepam is an active metabolite formed during the breakdown of diazepam, nordazepam, and certain similar drugs. It may be safer than many other benzodiazepines in patients with impaired liver function because it does not require hepatic oxidation, but rather, it is simply metabolized by glucuronidation, so oxazepam is less likely to accumulate and cause adverse reactions in the elderly or people with liver disease. Oxazepam is similar to lorazepam in this respect.[28] Preferential storage of oxazepam occurs in some organs, including the heart of the neonate. Absorption by any administered route and the risk of accumulation is significantly increased in the neonate, and withdrawal of oxazepam during pregnancy and breast feeding is recommended, as oxazepam is excreted in breast milk.[29]
2 mg of oxazepam equates to 1 mg of diazepam according to the benzodiazepine equivalency converter, therefore 20 mg of oxazepam according to BZD equivalency equates to 10 mg of diazepam and 15 mg oxazepam to 7.5 mg diazepam (rounded up to 8 mg of diazepam).[30] Some BZD equivalency converters use 3 to 1 (oxazepam to diazepam), 1 to 3 (diazepam to oxazepam) as the ratio (3:1 and 1:3), so 15 mg of oxazepam would equate to 5 mg of diazepam.[31]
Chemistry
Oxazepam exists as a racemic mixture.[32] Early attempts to isolate enantiomers were unsuccessful; the corresponding acetate has been isolated as a single enantiomer. Given the different rates of epimerization that occur at different pH levels, it was determined that there would be no therapeutic benefit to the administration of a single enantiomer over the racemic mixture.[33]
Frequency of use
Oxazepam, along with diazepam, nitrazepam, and temazepam, were the four benzodiazepines listed on the pharmaceutical benefits scheme and represented 82% of the benzodiazepine prescriptions in Australia in 1990–1991.[34] It is in several countries the benzodiazepine of choice for novice users, due to a low chance of accumulation and a relatively slow absorption speed.[35]
Society and culture
Misuse
Oxazepam has the potential for misuse, defined as taking the drug to achieve a high, or continuing to take the drug in the long term against medical advice.[36] Benzodiazepines, including diazepam, oxazepam, nitrazepam, and flunitrazepam, accounted for the largest volume of forged drug prescriptions in Sweden from 1982 to 1986. During this time, a total of 52% of drug forgeries were for benzodiazepines, suggesting they were a major prescription drug class of abuse.[37]
However, due to its slow rate of absorption and its slow onset of action,[27] oxazepam has a relatively low potential for abuse compared to some other benzodiazepines, such as temazepam, flunitrazepam, or triazolam, which have a high potential for abuse similar to barbiturates.[38]
Legal status
Oxazepam is a Schedule IV drug under the Convention on Psychotropic Substances.[39]
Brand names
It is marketed under many brand names worldwide, including: Alepam, Alepan, Anoxa, Anxiolit, Comedormir, durazepam, Murelax, Nozepam, Oksazepam, Opamox, Ox-Pam, Oxa-CT, Oxabenz, Oxamin, Oxapam, Oxapax, Oxascand, Oxaze, Oxazepam, Oxazépam, Oxazin, Oxepam, Praxiten, Purata, Selars, Serax, Serenal, Serepax, Seresta, Séresta, Serpax, Sobril, Tazepam, Vaben, and Youfei.[40]
It is also marketed in combination with hyoscine as Novalona and in combination with alanine as Pausafrent T.[40]
Environmental concerns
In 2013, a laboratory study which exposed European perch to oxazepam concentrations equivalent to those present in European rivers (1.8 micrograms liter–1) found that they exhibited increased activity, reduced sociality, and higher feeding rate.[41] In 2016, a follow-up study which exposed salmon smolt to oxazepam for seven days before letting them migrate observed increased intensity of migratory behaviour compared to controls.[42] A 2019 study associated this faster, bolder behaviour in exposed smolt to increased mortality due to a higher likelihood of being predated on.[43]
On the other hand, a 2018 study from the same authors, which kept 480 European perch and 12 northern pikes in 12 ponds over 70 days, half of them control and half spiked with oxazepam, found no significant difference in either perch growth or mortality. However, it suggested that the latter could be explained by the exposed perch and pike being equally hampered by oxazepam, rather than the lack of an overall effect.[44] Lastly, a 2021 study built on these results by comparing two whole lakes filled with perch and pikes - one control while the other was exposed to oxazepam 11 days into experiment, at concentrations between 11 and 24 μg L–1, which is 200 times greater than the reported concentrations in the European rivers. Even so, there were no measurable effects on pike behaviour after the addition of oxazepam, while the effects on perch behaviour were found to be negligible. The authors concluded that the effects previously attributed to oxazepam were instead likely caused by a combination of fish being stressed by human handling and small aquaria, followed by being exposed to a novel environment.[45]
References
- ↑
- 1 2 3 4 5 6 7 8 9 10 "Oxazepam Monograph for Professionals". Drugs.com. Archived from the original on 10 February 2018. Retrieved 10 November 2021.
- 1 2 3 4 5 6 7 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. X. ISBN 978-0857114105.
- ↑ "Encadré 1. Anxiolytiques à demi-vie courte (< 20 heures) et sans métabolite actif par ordre alphabétique de DCI" (PDF). Archived (PDF) from the original on 2021-06-28. Retrieved 2021-09-06.
- 1 2 Sonne, J; Loft, S; Døssing, M; Vollmer-Larsen, A; Olesen, KL; Victor, M; Andreasen, F; Andreasen, PB (1988). "Bioavailability and pharmacokinetics of oxazepam". European Journal of Clinical Pharmacology. 35 (4): 385–9. doi:10.1007/bf00561369. PMID 3197746. S2CID 31007311.
- ↑ Sonne, J; Boesgaard, S; Poulsen, H E; Loft, S; Hansen, J M; Døssing, M; Andreasen, F (November 1990). "Pharmacokinetics and pharmacodynamics of oxazepam and metabolism of paracetamol in severe hypothyroidism". British Journal of Clinical Pharmacology. 30 (5): 737–742. doi:10.1111/j.1365-2125.1990.tb03844.x. PMC 1368175. PMID 2271373.
- ↑ Fitzgerald, Margaret A. (14 March 2017). Nurse Practitioner Certification Examination and Practice Preparation. F.A. Davis. p. 346. ISBN 978-0-8036-6917-8. Archived from the original on 10 November 2021. Retrieved 10 November 2021.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 536. ISBN 9783527607495. Archived from the original on 2021-08-28. Retrieved 2021-09-06.
- ↑ "Oxazepam Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 April 2021. Retrieved 10 November 2021.
- ↑ Galanter, Marc; Kleber, Herbert D. (1 July 2008). The American Psychiatric Publishing Textbook of Substance Abuse Treatment (4th ed.). United States of America: American Psychiatric Publishing Inc. p. 216. ISBN 978-1-58562-276-4.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2011-07-15. Retrieved 2009-04-22.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Oxazepam Uses, Side Effects & Warnings - Drugs.com". drugs.com. Archived from the original on 2009-06-04.
- ↑ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM.; Eschalier, A. (November 2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Ann Pharm Fr. 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ↑ "FDA expands Boxed Warning to improve safe use of benzodiazepine drug". U.S. Food and Drug Administration (FDA). 23 September 2020. Archived from the original on 24 September 2020. Retrieved 23 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ MacKinnon GL; Parker WA. (1982). "Benzodiazepine withdrawal syndrome: a literature review and evaluation". The American Journal of Drug and Alcohol Abuse. 9 (1): 19–33. doi:10.3109/00952998209002608. PMID 6133446.
- ↑ McElhatton PR. (Nov–Dec 1994). "The effects of benzodiazepine use during pregnancy and lactation". Reprod Toxicol. 8 (6): 461–75. doi:10.1016/0890-6238(94)90029-9. PMID 7881198.
- ↑ Kanto JH. (May 1982). "Use of benzodiazepines during pregnancy, labour and lactation, with particular reference to pharmacokinetic considerations". Drugs. 23 (5): 354–80. doi:10.2165/00003495-198223050-00002. PMID 6124415. S2CID 27014006.
- ↑ Buckley NA, Dawson AH, Whyte IM, O'Connell DL (28 January 1995). "Relative toxicity of benzodiazepines in overdose". BMJ. 310 (6974): 219–21. doi:10.1136/bmj.310.6974.219. PMC 2548618. PMID 7866122.
- ↑ Gaudreault P, Guay J, Thivierge RL, Verdy I (1991). "Benzodiazepine poisoning. Clinical and pharmacological considerations and treatment". Drug Saf. 6 (4): 247–65. doi:10.2165/00002018-199106040-00003. PMID 1888441. S2CID 27619795.
- ↑ Perry HE, Shannon MW (June 1996). "Diagnosis and management of opioid- and benzodiazepine-induced comatose overdose in children". Current Opinion in Pediatrics. 8 (3): 243–7. doi:10.1097/00008480-199606000-00010. PMID 8814402. S2CID 43105029.
- ↑ Busto U, Kaplan HL, Sellers EM (February 1980). "Benzodiazepine-associated emergencies in Toronto". Am J Psychiatry. 137 (2): 224–7. doi:10.1176/ajp.137.2.224. PMID 6101526.
- ↑ Skerritt JH; Johnston GA. (May 6, 1983). "Enhancement of GABA binding by benzodiazepines and related anxiolytics". Eur J Pharmacol. 89 (3–4): 193–8. doi:10.1016/0014-2999(83)90494-6. PMID 6135616.
- ↑ Oelschläger H. (July 4, 1989). "[Chemical and pharmacologic aspects of benzodiazepines]". Schweiz Rundsch Med Prax. 78 (27–28): 766–72. PMID 2570451.
- ↑ Sonne, J; Boesgaard, S; Poulsen, H E; Loft, S; Hansen, J M; Døssing, M; Andreasen, F (November 1990). "Pharmacokinetics and pharmacodynamics of oxazepam and metabolism of paracetamol in severe hypothyroidism". British Journal of Clinical Pharmacology. 30 (5): 737–742. doi:10.1111/j.1365-2125.1990.tb03844.x. PMC 1368175. PMID 2271373.
- ↑ "Archive copy" (PDF). Archived (PDF) from the original on 2021-06-28. Retrieved 2021-09-06.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Christensen P; Lolk A; Gram LF; Kragh-Sørensen P. (1992). "Benzodiazepine-induced sedation and cortisol suppression. A placebo-controlled comparison of oxazepam and nitrazepam in healthy male volunteers". Psychopharmacology. 106 (4): 511–6. doi:10.1007/BF02244823. PMID 1349754. S2CID 29331503.
- 1 2 Serfaty M, Masterton G (1993). "Fatal poisonings attributed to benzodiazepines in Britain during the 1980s". Br J Psychiatry. 163 (3): 386–93. doi:10.1192/bjp.163.3.386. PMID 8104653. S2CID 46001278. Archived from the original on 2020-01-26. Retrieved 2021-09-06.
- ↑ Sabath, L. D. (1975). "Current status of treatment of pneumonia". Southern Medical Journal. 68 (12): 1507–11. doi:10.1097/00007611-197512000-00012. PMID 0792. S2CID 20789345.
- ↑ Olive G; Dreux C. (January 1977). "Pharmacologic bases of use of benzodiazepines in peréinatal medicine". Arch Fr Pediatr. 34 (1): 74–89. PMID 851373.
- ↑ "Archive copy". Archived from the original on 2019-05-13. Retrieved 2021-09-06.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Benzodiazepine equivalent dosage converter". GlobalRPH. Archived from the original on 2019-09-21. Retrieved 2019-09-21.
- ↑ Aso, Yukio; et al. (1988). "The Kinetics of the Racemization of Oxazepam in Aqueous Solution". Chemical and Pharmaceutical Bulletin. 36 (5): 1834–1840. doi:10.1248/cpb.36.1834. PMID 3203421.
- ↑ Crossley, Roger J. (1995). Chirality and Biological Activity of Drugs. CRC Press. ISBN 978-0849391408. Archived from the original on 2021-10-26. Retrieved 2021-09-06.
- ↑ Mant A; Whicker SD; McManus P; Birkett DJ; Edmonds D; Dumbrell D. (December 1993). "Benzodiazepine utilisation in Australia: report from a new pharmacoepidemiological database". Australian Journal of Public Health. 17 (4): 345–9. doi:10.1111/j.1753-6405.1993.tb00167.x. PMID 7911332.
- ↑ "Archive copy". Archived from the original on 2020-02-15. Retrieved 2021-09-06.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Griffiths RR, Johnson MW (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry. 66 Suppl 9: 31–41. PMID 16336040.
- ↑ Bergman U; Dahl-Puustinen ML. (1989). "Use of prescription forgeries in a drug abuse surveillance network". Eur. J. Clin. Pharmacol. 36 (6): 621–3. doi:10.1007/BF00637747. PMID 2776820. S2CID 19770310.
- ↑ Griffiths RR, Wolf B (August 1990). "Relative abuse liability of different benzodiazepines in drug abusers". J Clin Psychopharmacol. 10 (4): 237–43. doi:10.1097/00004714-199008000-00002. PMID 1981067. S2CID 28209526.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 2005-12-05. Retrieved 2005-11-19.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 "Oxazepam - International Brand Names". Drugs.com. Archived from the original on 5 January 2017. Retrieved 4 January 2017.
- ↑ Brodin T, Fick J, Jonsson M, Klaminder J (February 2013). "Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations". Science. 339 (6121): 814–5. doi:10.1126/science.1226850. PMID 23413353.
- ↑ Hellström G, Klaminder J, Finn F, Persson L, Alanärä A, Jonsson M, et al. (December 2016). "GABAergic anxiolytic drug in water increases migration behaviour in salmon". Nature Communications. 7 (13460): 13460. doi:10.1038/ncomms13460. PMC 5155400. PMID 27922016.
- ↑ Klaminder J, Jonsson M, Leander J, Fahlman J, Brodin T, Fick J, Hellström G (October 2019). "Less anxious salmon smolt become easy prey during downstream migration". The Science of the Total Environment. 687: 488–493. doi:10.1016/j.scitotenv.2019.05.488. PMID 31212157.
- ↑ Lagesson A, Brodin T, Fahlman J, Fick J, Jonsson M, Persson J, et al. (February 2018). "No evidence of increased growth or mortality in fish exposed to oxazepam in semi-natural ecosystems". The Science of the Total Environment. 615: 608–614. doi:10.1016/j.scitotenv.2017.09.070. PMID 28988097.
- ↑ Fahlman J, Hellström G, Jonsson M, Fick JB, Rosvall M, Klaminder J (March 2021). "Impacts of Oxazepam on Perch (Perca fluviatilis) Behavior: Fish Familiarized to Lake Conditions Do Not Show Predicted Anti-anxiety Response". Environmental Science & Technology. 55 (6): 3624–3633. doi:10.1021/acs.est.0c05587. PMID 33663207.
External links
| Identifiers: |
|---|
- Inchem - Oxazepam Archived 2020-02-19 at the Wayback Machine