FG-8205
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
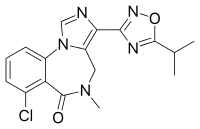
| Formula | C17H16ClN5O2 |
| Molar mass | 357.80 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
FG-8205[1] (L-663,581) is an imidazobenzodiazepine derivative related to bretazenil, which acts as a partial agonist at GABAA receptors, with slight selectivity for the α1-containing subtype. In animal tests it has anxiolytic and anticonvulsant effects but with little sedation or ataxia produced.[2][3][4][5]
See also
References
- ↑ Watjen F, Baker R, Engelstoff M, Herbert R, MacLeod A, Knight A, Merchant K, Moseley J, Saunders J, Swain CJ (October 1989). "Novel benzodiazepine receptor partial agonists: oxadiazolylimidazobenzodiazepines". J. Med. Chem. 32 (10): 2282–91. doi:10.1021/jm00130a010. PMID 2552115.
- ↑ Tricklebank MD, Honoré T, Iversen SD, Kemp JA, Knight AR, Marshall GR, Rupniak NM, Singh L, Tye S, Watjen F (November 1990). "The pharmacological properties of the imidazobenzodiazepine, FG 8205, a novel partial agonist at the benzodiazepine receptor". Br. J. Pharmacol. 101 (3): 753–61. doi:10.1111/j.1476-5381.1990.tb14152.x. PMC 1917729. PMID 1963808.
- ↑ Lin JH, Chen IW, Lin TH (December 1994). "Blood–brain barrier permeability and in vivo activity of partial agonists of benzodiazepine receptor: a study of L-663,581 and its metabolites in rats". J. Pharmacol. Exp. Ther. 271 (3): 1197–202. PMID 7996426.
- ↑ Guscott MR, Cook GP, Bristow LJ (September 2000). "Contextual fear conditioning and baseline startle responses in the rat fear-potentiated startle test: a comparison of benzodiazepine/gamma-aminobutyric acid-A receptor agonists". Behav Pharmacol. 11 (6): 495–504. doi:10.1097/00008877-200009000-00006. PMID 11103915. S2CID 33999227.
- ↑ Atack JR (August 2003). "Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site". Curr Drug Targets CNS Neurol Disord. 2 (4): 213–32. doi:10.2174/1568007033482841. PMID 12871032.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.