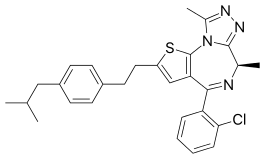
Israpafant
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C28H29ClN4S |
| Molar mass | 489.08 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Israpafant (Y-24180) is a drug which acts as a selective antagonist for the platelet-activating factor receptor,[1] and was originally developed for the treatment of asthma.[2] Its chemical structure is a thienotriazolodiazepine, closely related to the sedative benzodiazepine derivative etizolam. However israpafant binds far more tightly to the platelet-activating factor receptor, with an IC50 of 0.84nM for inhibiting PAF-induced human platelet aggregation (compared to etizolam's IC50 of 998nM at this target), while it binds only weakly to benzodiazepine receptors, with a Ki of 3680nM.[3] Israpafant has been found to inhibit the activation of eosinophil cells,[4][5][6] and consequently delays the development of immune responses. It has also been shown to have anti-nephrotoxic properties,[7] and to mobilize calcium transport.[8]
See also
References
- ↑ Hirota N, Yasuda D, Hashidate T, Yamamoto T, Yamaguchi S, Nagamune T, et al. (February 2010). "Amino acid residues critical for endoplasmic reticulum export and trafficking of platelet-activating factor receptor". The Journal of Biological Chemistry. 285 (8): 5931–40. doi:10.1074/jbc.M109.066282. PMC 2820818. PMID 20007715.
- ↑ Hozawa S, Haruta Y, Ishioka S, Yamakido M (October 1995). "Effects of a PAF antagonist, Y-24180, on bronchial hyperresponsiveness in patients with asthma". American Journal of Respiratory and Critical Care Medicine. 152 (4 Pt 1): 1198–202. doi:10.1164/ajrccm.152.4.7551370. PMID 7551370.
- ↑ Takehara S, Mikashima H, Muramoto Y, Terasawa M, Setoguchi M, Tahara T (December 1990). "Pharmacological actions of Y-24180, a new specific antagonist of platelet activating factor (PAF): II. Interactions with PAF and benzodiazepine receptors". Prostaglandins. 40 (6): 571–83. doi:10.1016/0090-6980(90)90002-D. PMID 1965554.
- ↑ Komatsu H, Amano M, Yamaguchi S, Sugahara K (1999). "Inhibition of activation of human peripheral blood eosinophils by Y-24180, an antagonist to platelet-activating factor receptor". Life Sciences. 65 (13): PL171-6. doi:10.1016/s0024-3205(99)00385-9. PMID 10503965.
- ↑ Mizuki M, Komatsu H, Akiyama Y, Iwane S, Tsuda T (1999). "Inhibition of eosinophil activation in bronchoalveolar lavage fluid from atopic asthmatics by Y-24180, an antagonist to platelet-activating factor". Life Sciences. 65 (20): 2031–9. doi:10.1016/s0024-3205(99)00470-1. PMID 10579457.
- ↑ Satoh T, Tahara E, Yamada T, Watanabe C, Itoh T, Terasawa K, et al. (February 2000). "Differential effect of antiallergic drugs on IgE-mediated cutaneous reaction in passively sensitized mice". Pharmacology. 60 (2): 97–104. doi:10.1159/000028353. PMID 10657759. S2CID 6264992.
- ↑ Kawaguchi A, Sugimoto K, Fujimura A (January 2001). "Preventive effect of platelet-activating factor antagonist, Y-24180, against cyclosporine-induced acute nephrotoxicity". Life Sciences. 68 (10): 1181–90. doi:10.1016/s0024-3205(00)01028-6. PMID 11228102.
- ↑ Chao YY, Jan CR (January 2004). "Effect of Y-24180 on Ca2+ movement and proliferation in renal tubular cells". Life Sciences. 74 (7): 923–33. doi:10.1016/j.lfs.2003.09.033. PMID 14659980.