Uldazepam
 | |
| Clinical data | |
|---|---|
| Other names | 7-chloro-5-(2-chlorophenyl)-N-prop-2-enoxy-3H-1,4-benzodiazepin-2-amine |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H15Cl2N3O |
| Molar mass | 360.24 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Uldazepam is a drug which is a benzodiazepine derivative.[1] It has sedative and anxiolytic effects similar to those of other benzodiazepines.[2][3]
Synthesis
Thio thionamide is even more prone to amidine formation than the lactam itself.
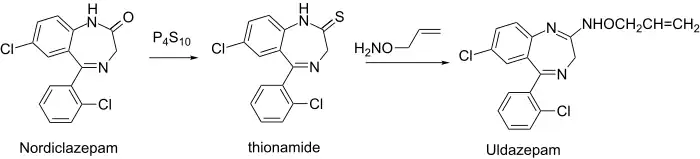
Uldazepam synthesis:[4] J. B. Hester, Jr., (1970); Chem. Abstr., 73: 99,001t (1970).
Reaction of thionamide (2) with O-allyl-hydroxylamine gave the oximino (3) uldazepam.
See also
References
- ↑ "Uldazepam U 31920". Psychotropics. Retrieved 12 June 2013.
- ↑ Oelschläger H, Ellaithy MM, Volke J (February 1988). "[Mechanism of the polarographic reduction of the tranquilizer uldazepam]". Archiv der Pharmazie (in German). 321 (2): 69–72. doi:10.1002/ardp.19883210205. PMID 3369929. S2CID 96356746.
- ↑ Itil TM, Akpinar S, Ozkut H, Balki N, Herrmann WM (June 1974). "Clinical and computerized EEG effects of U-31,920, a new anxiolytic". Current Therapeutic Research, Clinical and Experimental. 16 (6): 642–54. PMID 4211146.
- ↑ DE 2005176, Hester Jr., Jackson Boling, "3H-1,4-benzodiazepine und Verfahren zu deren Herstellung [3H-1,4-benzodiazepines and processes for their preparation]", published 1970-09-10, assigned to The Upjohn Co.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.