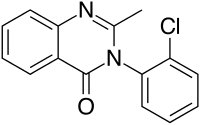
Mecloqualone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.848 |
| Chemical and physical data | |
| Formula | C15H11ClN2O |
| Molar mass | 270.714 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mecloqualone (Nubarene, Casfen) is a Quinazolinone-class GABAergic and is an analogue of methaqualone that was first made in 1960[1] and marketed mainly in France and some other European countries. It has sedative, hypnotic, and anxiolytic properties caused by its agonist activity at the β subtype of the GABAa receptor, and was used for the treatment of insomnia.[2] Mecloqualone is faster-acting but shorter-lasting than methaqualone and so was used only as a sleeping pill,[3] in contrast to methaqualone, which was used as a general-purpose anxiolytic as well. Mecloqualone was never as widely used as methaqualone and is no longer prescribed because of concerns about its potential for abuse and overdose. In the United States it is a Schedule I non-narcotic (depressant) controlled substance with an ACSCN of 2572 and zero annual aggregate manufacturing quota.
See also
References
- ↑ Jackman GB, Petrow V, Stephenson O (September 1960). "Some 2, 3-disubstituted 3H-4-quinazolones and 3H-4-thioquinazolones". The Journal of Pharmacy and Pharmacology. 12: 529–38. doi:10.1111/j.2042-7158.1960.tb12705.x. PMID 14406263. S2CID 31254238.
- ↑ Mouren P, Giraud F, Pinsard N (1963). "[Clinical use of a new psycholeptic: Mecloqualone]". Marseille Medical. 100: 599–602. PMID 13936358.
- ↑ Dubnk B, Towne CA, Bush MT (November 1969). "Detection, assay and rate of excretion of mecloqualone in animals and man". Toxicology and Applied Pharmacology. 15 (3): 632–41. doi:10.1016/0041-008X(69)90065-9. PMID 5353825.