Seproxetine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 4–16 days |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
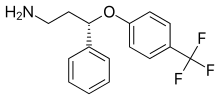
| Formula | C16H16F3NO |
| Molar mass | 295.305 g·mol−1 |
Seproxetine, also known as (S)-norfluoxetine, is a selective serotonin reuptake inhibitor (SSRI).[1] It is the S enantiomer of norfluoxetine, the main active metabolite of the widely used antidepressant fluoxetine; but little is known about its pharmacological actions. Seproxetine was being investigated by Eli Lilly and Company as an antidepressant; however, cardiac side effects were discovered and development was discontinued.[1]
References
- 1 2 "Seproxetine". DrugBank. University of Alberta. Retrieved 10 August 2016.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| DAT (DRIs) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NET (NRIs) |
| ||||||||||||||
| SERT (SRIs) |
| ||||||||||||||
| VMATs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Corporate directors |
|
|---|---|
| Products |
|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.