PPPA (drug)
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
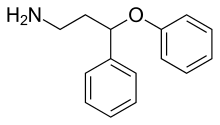
| Formula | C15H17NO |
| Molar mass | 227.307 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
PPPA, or 3-phenoxy-3-phenylpropan-1-amine, is a drug which is described as an antidepressant.[1] It was derived by Eli Lilly from the antihistamine diphenhydramine, a 2-diphenylmethoxyethanamine derivative with additional properties as a selective serotonin reuptake inhibitor (SSRI), and has been the basis for the subsequent discovery of a number of other antidepressant drugs.[2][3][4]
List of PPPA derivatives
- Atomoxetine ((3R)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine) — NRI[1]
- Fluoxetine (N-methyl-3-(4-(trifluoromethyl)phenoxy)-3-phenylpropan-1-amine) — SSRI[2]
- N-Methyl-PPPA (N-methyl-3-phenoxy-3-phenylpropan-1-amine) — SNRI[2][4]
- Nisoxetine (N-methyl-3-(2-methoxyphenoxy)-3-phenylpropan-1-amine) — NRI[1]
- Norfluoxetine (3-(4-(trifluoromethyl)phenoxy)-3-phenylpropan-1-amine) — SSRI[3]
- Seproxetine ((S)-3-(4-(trifluoromethyl)phenoxy)-3-phenylpropan-1-amine) — SSRI[5]
Structurally related drugs include dapoxetine, duloxetine, edivoxetine, femoxetine, paroxetine, reboxetine, and viloxazine, all of which act, similarly, as monoamine reuptake inhibitors, and most of which are, again similarly, antidepressants.[1][3]
Zimelidine is an antidepressant and SSRI which was derived from the antihistamine pheniramine, which, similarly to its analogues brompheniramine and chlorpheniramine, possesses SNRI properties.[4] Fluvoxamine, another antidepressant and SSRI, was developed from the antihistamine tripelennamine, which possesses SNDRI actions.[6]
See also
References
- 1 2 3 4 Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 414–. ISBN 978-0-7817-6879-5.
- 1 2 3 Francisco Lopez-Munoz; Cecilio Alamo (9 September 2011). Neurobiology of Depression. CRC Press. pp. 132–. ISBN 978-1-4398-3850-1.
- 1 2 3 Janos Fischer; C. Robin Ganellin (24 August 2010). Analogue-based Drug Discovery II. John Wiley & Sons. pp. 35, 282, 284. ISBN 978-3-527-63212-1.
- 1 2 3 Walter Sneader (31 October 2005). Drug Discovery: A History. John Wiley & Sons. pp. 416–417. ISBN 978-0-470-01552-0.
- ↑ David G. Watson (9 February 2011). Pharmaceutical Chemistry. Elsevier Health Sciences. pp. 1061–. ISBN 978-0-7020-4850-0.
- ↑ David Healy (1 June 2004). Let Them Eat Prozac: The Unhealthy Relationship Between the Pharmaceutical Industry and Depression. NYU Press. pp. 295–. ISBN 978-0-8147-7300-0.
Further reading
- Wong DT, Bymaster FP, Engleman EA (1995). "Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication". Life Sci. 57 (5): 411–41. doi:10.1016/0024-3205(95)00209-o. PMID 7623609.