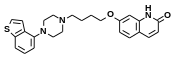
Brexpiprazole
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /brɛkˈspɪprəzoʊl/ brek-SPIP-rə-zohl /rɛkˈsʌlti/ rek-SUL-tee |
| Trade names | Rexulti, Rxulti |
| Other names | OPC-34712 |
IUPAC name
| |
| Clinical data | |
| Drug class | Atypical antipsychotic[1] |
| Main uses | Schizophrenia, major depressive disorder[1] |
| Side effects | Weight gain, movement disorder[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | By mouth (tablets) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615046 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 95% (Tmax = 4 hours)[4] |
| Protein binding | >99% |
| Metabolism | Liver (mainly mediated by CYP3A4 and CYP2D6) |
| Elimination half-life | 91 hours (brexpiprazole), 86 hours (major metabolite) |
| Excretion | Feces (46%), urine (25%) |
| Chemical and physical data | |
| Formula | C25H27N3O2S |
| Molar mass | 433.57 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Brexpiprazole, sold under the brand name Rexulti among others, is an atypical antipsychotic used to treat schizophrenia, and major depressive disorder (MDD).[1] In schizophrenia it may be used in those over the age of 12.[1] It is taken by mouth.[5]
Common side effects include weight gain and a movement disorder known as akathisia.[1] Other side effects may include neuroleptic malignant syndrome, tardive dyskinesia, diabetes, problem gambling, and low white blood cells.[1] Use in older people with dementia increases the risk of death.[1] Safety in pregnancy is unclear.[6] It is believed to work by affecting dopamine, serotonin, and noradrenaline in the brain.[5]
Brexpiprazole was approved for medical use in the United States in 2015 and Europe in 2018.[1][5] It is not available in the United Kingdom as of 2022.[7] In the United States it costs about 1,200 USD per month as of 2022, regardless of the dose.[8]
Medical uses
Brexpiprazole is used in the treatment of schizophrenia and as an adjunct for major depressive disorder.[9]
Dosage
It is started at a dose of 0.5 to 1 mg per day, which may be increased to a maximum of 4 mg per day.[1]
Side effects
The most common adverse events associated with brexpiprazole (all doses of brexpiprazole cumulatively greater than or equal to 5% vs. placebo) were upper respiratory tract infection (6.9% vs. 4.8%), akathisia (6.6% vs. 3.2%), weight gain (6.3% vs. 0.8%), and nasopharyngitis (5.0% vs. 1.6%).[10]
Interactions
Based on information given on the consent forms, it seems brexpiprazole is a substrate of CYP2D6 and CYP3A4, like its predecessor aripiprazole. Participants in the clinical trials are advised to avoid grapefruit, Seville oranges and related citruses.
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Ref |
|---|---|---|---|
| 5-HT1A | 0.12 | Partial agonist | [12] |
| 5-HT1B | 32 | ND | [12] |
| 5-HT2A | 0.47 | Antagonist | [12] |
| 5-HT2B | 1.9 | Antagonist | [12] |
| 5-HT2C | 12–34 | Partial agonist | [12] |
| 5-HT5A | 140 | ND | [12] |
| 5-HT6 | 58 | Antagonist | [12] |
| 5-HT7 | 3.7 | Antagonist | [12] |
| D1 | 160 | ND | [12] |
| D2L | 0.30 | Partial agonist | [12] |
| D3 | 1.1 | Partial agonist | [12] |
| D4 | 6.3 | ND | [12] |
| D5 | ND | ND | ND |
| α1A | 3.8 | Antagonist | [12] |
| α1B | 0.17 | Antagonist | [12] |
| α1D | 2.6 | Antagonist | [12] |
| α2A | 15 | Antagonist | [12] |
| α2B | 17 | Antagonist | [12] |
| α2C | 0.59 | Antagonist | [12] |
| β1 | 59 | Antagonist | [12] |
| β2 | 67 | Antagonist | [12] |
| β3 | >10,000 | ND | [12] |
| H1 | 19 | Antagonist | [12] |
| H2 | >10,000 | ND | [12] |
| H3 | >10,000 | ND | [12] |
| mACh | 52% at 10 μM | ND | [12] |
| M1 | 67% at 10 μM | ND | [12] |
| M2 | >10,000 | ND | [12] |
| σ | 96% at 10 μM | ND | [12] |
| SERT | 65% at 10 μM | Blocker | [12] |
| NET | 0% at 10 μM | Blocker | [12] |
| DAT | 90% at 10 μM | Blocker | [12] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. Most or all data are for human cloned proteins. | |||
Brexpiprazole acts as a partial agonist of the serotonin 5-HT1A receptor and the dopamine D2 and D3 receptors.[12] Partial agonists have both blocking properties and stimulating properties at the receptor they bind to. The ratio of blocking activity to stimulating activity determines a portion of its clinical effects. Brexpiprazole has more blocking and less stimulating activity at the dopamine receptors than its predecessor, aripiprazole, which may decrease its risk for agitation and restlessness.[12] Specifically, where aripiprazole has an intrinsic activity or agonist effect at the D2 receptor of 60%+, brexpiprazole has an intrinsic activity at the same receptor of about 45%. For aripiprazole, this means more dopamine receptor activation at lower doses, with blockade being reached at higher doses, whereas brexpiprazole is the opposite. By contrast, brexpiprazole has a much higher affinity for the 5-HT1A receptor than aripiprazole as well as a much higher intrinsic activity. In vivo characterization of brexpiprazole shows that it may act as a near-full agonist of the 5-HT1A receptor. This may further underlie a lower potential than aripiprazole to cause treatment-emergent, movement-related disorders such as akathisia due to the downstream dopamine release that is triggered by 5-HT1A receptor agonism. It is also an antagonist of the serotonin 5-HT2A, 5-HT2B, and 5-HT7 receptors, which may contribute to antidepressant effect. It also binds to and blocks the α1A-, α1B-, α1D-, and α2C-adrenergic receptors.[12] The drug has negligible affinity for the muscarinic acetylcholine receptors, and hence has no anticholinergic effects.[12] Although brexpiprazole has less affinity for H1 compared to aripiprazole weight gain can occur.[13]
History
In November 2011, Otsuka Pharmaceutical and Lundbeck announced a global alliance.[14] Lundbeck gave Otsuka an upfront payment of $200 million, and the deal includes development, regulatory and sales payments, for a potential total of $1.8 billion.
Specifically for OPC-34712, Lundbeck will obtain 50% of net sales in Europe and Canada and 45% of net sales in the US from Otsuka.
Society and culture
Legal status
In January 2018, it was approved for the treatment of schizophrenia in Japan.[15]
Patents
- U.S. Patent 8,071,600
- WIPO PCT/JP2006/317704
- Canadian patent: 2620688[16]
Research
Brexpiprazole was under development for the treatment of attention deficit hyperactivity disorder (ADHD) as an adjunct to stimulants, but was discontinued for this indication.[17][18][19] It reached phase 2 clinical trials for this use prior to discontinuation.[19]
References
- 1 2 3 4 5 6 7 8 9 10 "DailyMed - REXULTI- brexpiprazole tablet". dailymed.nlm.nih.gov. Archived from the original on 11 May 2021. Retrieved 11 January 2022.
- 1 2 "Brexpiprazole (Rexulti) Use During Pregnancy". Drugs.com. 10 February 2020. Archived from the original on 5 December 2020. Retrieved 18 August 2020.
- ↑ "Summary for ARTG Entry:273224 Rexulti brexpiprazole 4 mg film coated tablets blisters". Therapeutic Goods Administration (TGA). Retrieved 18 August 2020.
{{cite web}}: CS1 maint: url-status (link) - ↑ "REXULTI® (brexpiprazole) Tablets, for Oral Use. Full Prescribing Information" (PDF). Rexulti (brexpiprazole) Patient Site. Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan. Archived (PDF) from the original on 5 March 2016. Retrieved 15 July 2015.
- 1 2 3 "Rxulti". Archived from the original on 12 January 2022. Retrieved 11 January 2022.
- ↑ "Brexpiprazole (Rexulti) Use During Pregnancy". Drugs.com. Archived from the original on 5 December 2020. Retrieved 11 January 2022.
- ↑ "Brexpiprazole". SPS - Specialist Pharmacy Service. 10 February 2016. Archived from the original on 12 January 2022. Retrieved 11 January 2022.
- ↑ "Brexpiprazole Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 11 January 2022.
- ↑ "FDA approves new drug to treat schizophrenia and as an add on to an antidepressant to treat major depressive disorder". U.S. Food and Drug Administration (FDA) Newsroom (Press release). FDA. 2015-07-13. Archived from the original on 2015-07-15. Retrieved 14 July 2015.
- ↑ "Otsuka Pharmaceutical reports OPC-34712 Phase 2 trial results in major depressive disorder". News-Medical.Net. 2011-05-16. Archived from the original on 2021-11-04. Retrieved 10 February 2012.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 9 January 2021. Retrieved 14 August 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, et al. (September 2014). "Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator". The Journal of Pharmacology and Experimental Therapeutics. 350 (3): 589–604. doi:10.1124/jpet.114.213793. PMID 24947465. S2CID 10768032.
- ↑ Stahl SM (February 2016). "Mechanism of action of brexpiprazole: comparison with aripiprazole". CNS Spectrums. 21 (1): 1–6. doi:10.1017/S1092852915000954. PMID 26899451.
- ↑ "Lundbeck and Otsuka Pharmaceutical sign historic agreement to deliver innovative medicines targeting psychiatric disorders worldwide". Lundbeck. Archived from the original on 1 April 2012. Retrieved 10 February 2012.
- ↑ "Otsuka Receives Approval in Japan for the Manufacture and Sale of New Antipsychotic Drug Rexulti Tablets for Schizophrenia|News Releases". Otsuka Pharmaceutical Co., Ltd. Archived from the original on 2018-10-04. Retrieved 2018-10-04.
- ↑ "Canadian Patents Database 2620688". Archived from the original on 4 March 2016. Retrieved 16 February 2012.
- ↑ "Brexpiprazole - Lundbeck/Otsuka - AdisInsight". Archived from the original on 2021-09-22. Retrieved 2021-10-20.
- ↑ Howland RH (April 2015). "Brexpiprazole: another multipurpose antipsychotic drug?". J Psychosoc Nurs Ment Health Serv. 53 (4): 23–5. doi:10.3928/02793695-20150323-01. PMID 25856810.
- 1 2 "A Phase 2, Multicenter, Randomized, Double-blind, Placebo Controlled Study of the Safety and Efficacy of OPC-34712 as Adjunctive Therapy in the Treatment of Adult Attention Deficit/ Hyperactivity Disorder". 23 October 2012. Archived from the original on 22 September 2021. Retrieved 20 October 2021.
{{cite journal}}: Cite journal requires|journal=(help)
Further reading
- "Australian Public Assessment Report for Brexpiprazole". Therapeutic Goods Administration (TGA). September 2018. Archived from the original on 2021-11-04. Retrieved 2021-10-20.
External links
| External sites: |
|
|---|---|
| Identifiers: |