Nemonapride
 | |
| Clinical data | |
|---|---|
| Trade names | Emilace (JP) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
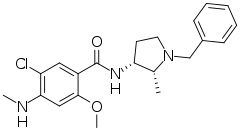
| Formula | C21H26ClN3O2 |
| Molar mass | 387.91 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Nemonapride (エミレース, Emilace (JP)) is an atypical antipsychotic approved in Japan for the treatment of schizophrenia. It was launched by Yamanouchi in 1991. Nemonapride acts as a D2 and D3 receptor antagonist, and is also a potent 5-HT1A receptor agonist. It has affinity for sigma receptors.
See also
- Benzamide
References
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.