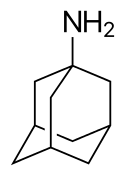
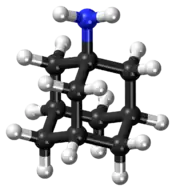
Amantadine
 | |
 | |
| Names | |
|---|---|
| Trade names | Gocovri, Symadine, Symmetrel, others |
| Other names | 1-Adamantylamine |
IUPAC name
| |
| Clinical data | |
| Main uses | Movement disorders, post herpetic neuralgia, tiredness from multiple sclerosis[1][2] |
| Side effects | Nausea, lightheadedness, trouble sleeping[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682064 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 86–90%[3] |
| Protein binding | 67%[3] |
| Metabolism | Minimal (mostly to acetyl metabolites)[3] |
| Elimination half-life | 10–31 hours[3] |
| Excretion | Urine[3] |
| Chemical and physical data | |
| Formula | C10H17N |
| Molar mass | 151.253 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Amantadine, sold under the brand name Gocovri among others, is a medication used to treat movement disorders associated with parkinsonism and antipsychotic medication.[2] Other uses may include post herpetic neuralgia and tiredness from multiple sclerosis.[1] It was used to treat and prevent seasonal influenza type A; however, since resistance developed in 2005 such use is no longer recommended.[2] It is taken by mouth.[2]
Amantadine is generally well tolerated.[5] Common side effects include nausea, lightheadedness, and trouble sleeping.[2] Other side effects include swelling of the legs, dry mouth, constipation, neuroleptic malignant syndrome, thoughs of suicide, and hallucinations.[5] Smaller doses are required in people with kidney problems.[2] Use is not recommended during pregnancy or breastfeeding.[1] How it works in movement disorders is unclear, but is believed to be related to increase dopamine effects.[2]
Amantadine was approved for medical use in the United States in 1968.[2] It is available as a generic medication.[1] In the United Kingdom 56 tablets of 100 mg costs the NHS about 36 pounds in 2020.[1] This amount in the United States costs about 23 USD.[6]
Medical uses
Parkinson's
Amantadine is used to treat Parkinson's disease-related dyskinesia and drug-induced parkinsonism syndromes.[7] Amantadine may be used alone or in combination with another anti-Parkinson or anticholinergic drug.[8] The specific symptoms targeted by amantadine therapy are dyskinesia and rigidity. The extended release amantadine formulation is commonly used to treat dyskinesias in patients with Parkinson disease receiving levodopa therapy.[8][9]
A 2003 Cochrane review concluded evidence inadequately proved the safety or efficacy of amantadine to treat dyskinesia.[10]
The World Health Organization in 2008, reported amantadine is not effective as a stand-alone parkinsonian therapy. Amantadine was recommended for combination therapy with levodopa.[11]
An extended release formulation is used to treat levodopa-induced dyskinesia in Parkinson's.[12]The WHO recommends the use of amantadine as a combination therapy to reduce levodopa side effects.[11]
Influenza A
Amantadine is no longer recommended for treatment or prophylaxis of influenza A in the United States.[13] Amantadine has no effect preventing or treating influenza B infections.[13] The US Centers for Disease Control and Prevention found 100% of seasonal H3N2 and 2009 pandemic flu samples were resistant to adamantanes (amantadine and rimantadine) during the 2008–2009 flu season.[8][14]
The CDC guidelines updated to recommend only neuraminidase inhibitors for influenza treatment and prophylaxis. The CDC currently recommends against amantadine and rimantadine to treat influenza A infections.[13]
Similarly, the 2011 World Health Organization virology report showed all tested H1N1 influenza A viruses were resistant to amantadine.[15] Current WHO guidelines recommend against use of M2 inhibitors for influenza A. The continued high rate of resistance observed in laboratory testing of influenza A has reduced the priority of M2 resistance testing.
A 2014 Cochrane review did not find evidence for benefit or safety of amantadine for the prevention or treatment of influenza A.[16]
Fatigue
Amantadine may be used for fatigue due to multiple sclerosis.[1][17] A 2007 Cochrane review concluded that there was no overall evidence supporting such use.[18] A follow up 2012 Cochrane review stated that there may be some amantadine-induced improvement in fatigue in some people with MS.[19] Despite multiple control trials that have also demonstrated improvements in subjective and objective ratings of fatigue, there is no final conclusion regarding its effectiveness.[20]
Consensus guidelines from the German Multiple Sclerosis Society (GMSS) in 2006 state that amantadine produces moderate improvement in subjective fatigue, problem solving, memory, and concentration. Thus, GMSS guidelines strongly recommend the use of amantadine in MS-related fatigue.[21]
Disorders of consciousness
Disorders of consciousness (DoC) include coma, vegetative state (VS), and minimally conscious state (MCS). Amantadine has been shown to increase the rate of emergence from a MCS, defined by consistent demonstration of interactive communication and functional objective use. In traumatic brain injury patients in the intensive care unit, amantadine has also been shown in various randomized control trials to increase the rate of functional recovery and arousal, particularly in the time period immediately following an injury.[22] In 2018 the American Academy of Neurology (AAN) updated treatment guidelines on the use of amantadine for prolonged DoC, recommending the use of amantadine (100-200 mg bid) for adults with DoC 4-16 weeks post injury to support early functional recovery and reduce disability.[23]
Brain injury
There is tentative evidence for amantadine helping with recovery from a brain injury.[24] The time-limited window following a brain injury is characterized by neuroplasticity, or the capacity of neurons in the brain to adapt and compensate after injury. Thus, physiatrists will often start patients on amantadine as soon as impairments are recognized. There are also case reports showing improved functional recovery with amantadine treatment occurring years after the initial brain injury.[24] There is insufficient evidence to determine if the functional gains are a result of effects through the dopamine or norepinephrine pathways. Some patients may benefit from direct dopamine stimulation with amantadine, while others may benefit more from other stimulants that act more on the norepinephrine pathway, such as methylphenidate.[24] It is unclear if treatment with amantadine improves long-term outcomes or simply accelerates recovery.[25]
Dosage
For parkinsonism the typically dose is 100 mg a day for one week and than 100 mg twice per day.[1] Doses as high as 400 mg per day may occasionally be used.[1]
Side effects
Amantadine is generally well tolerated and has a mild side-effect profile.[26] It should also be taken with caution in those with enlarged prostates or glaucoma, due to its anticholinergic effects.[27]
Neurological
Side effects include drowsiness (especially while driving), light headedness, falls, and dizziness.[28] Patients on Amantadine should avoid combination with other central nervous system (CNS) depressing agents, such as alcohol. Excessive alcohol usage may increase the potential for CNS effects such as dizziness, confusion, and light headedness.[29]
Rare severe adverse effects include neuroleptic malignant syndrome, depression, convulsions, psychosis, and suicidal ideation.[29] It has also been associated with inhibited actions (gambling, sexual activity, spending, other addictions) and diminished control over compulsions.[28]
Cardiovascular
Amantadine may cause orthostatic hypotension, syncope, and peripheral edema.[28]
Gastrointestinal
Amantadine has also been associated with dry mouth and constipation.[28]
Skin
Rare cases of skin rashes, such as Stevens–Johnson syndrome and livedo reticularis have also been reported in patients treated with Amantadine.[30][31]
Pregnancy and breastfeeding
Amantadine is Food and Drug Administration category C for pregnancy. Teratogenic effects have been observed in humans (case reports) and animal reproduction studies. Amantadine may also be present in breast milk and negatively alter breast milk production or excretion. The decision to breastfeed during amantadine therapy should consider the risk of infant exposure, the benefits of breastfeeding, and the benefits of the drug to the mother.[29]
Interactions
Amantadine may affect the central nervous system due to dopaminergic and anticholinergic properties. The mechanisms of action are not fully known. Because of the CNS effects, caution is required when prescribing additional CNS stimulants or anticholinergic drugs.[32] Thus, concurrent use of alcohol with Amantadine is not recommended due to enhanced CNS depressant effects.[33] In addition, anti-dopaminergic drugs such as metoclopramide and typical anti-psychotics should be avoided.[34][35] These interactions are likely related to opposing dopaminergic mechanisms of action, which inhibits amantadine's anti-Parkinson effects.
Contraindications
Amantadine is contraindicated in persons with end stage renal disease.[12] The drug is renally cleared.[36][32][37]
Amantadine may have anticholinergic side effects. Thus, patients with an enlarged prostate or glaucoma should use with caution. [38]
Live attenuated vaccines are contraindicated while taking amantadine.[12] It is possible that amantadine will inhibit viral replication and reduce the efficacy of administered vaccines. The U.S. Food and Drug Administration recommends avoiding amantadine for two weeks prior to vaccine administration and 48 hours afterwards.[32]
Mechanism of action
Parkinson's disease
The mechanism of its antiparkinsonian effect is poorly understood.[39] Amantadine is a weak antagonist of the NMDA-type glutamate receptor, increases dopamine release, and blocks dopamine reuptake.[40][41][42] Amantadine probably does not inhibit monoamine oxidase (MAO) enzyme.[43] Moreover, the drug has many effects in the brain, including release of dopamine and norepinephrine from nerve endings. It appears to be an anticholinergic (specifically at alpha-7 nicotinic receptors) like the similar pharmaceutical memantine.
In 2004, it was discovered that amantadine and memantine bind to and act as agonists of the σ1 receptor (Ki = 7.44 μM and 2.60 μM, respectively), and that activation of the σ1 receptor is involved in the dopaminergic effects of amantadine at therapeutically relevant concentrations.[44] These findings may also extend to the other adamantanes such as adapromine, rimantadine, and bromantane, and could explain the psychostimulant-like effects of this family of compounds.[44]
Influenza
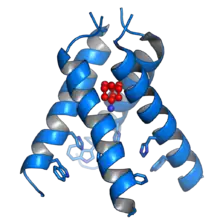

The mechanisms for amantadine's antiviral and antiparkinsonian effects are unrelated.[36][32] Amantadine targets the influenza A M2 ion channel protein. The M2 protein's function is to allow the intracellular virus to replicate (M2 also functions as a proton channel for hydrogen ions to cross into the vesicle), and exocytose newly formed viral proteins to the extracellular space (viral shedding). By blocking the M2 channel, the virus is unable to replicate because of impaired replication, protein synthesis, and exocytosis.[46]
Amantadine and rimantadine function in a mechanistically identical fashion, entering the barrel of the tetrameric M2 channel and blocking pore function—i.e., proton translocation.[8]
Resistance to the drug class is a consequence of mutations to the pore-lining amino acid residues of the channel, preventing both amantadine and rimantadine from binding and inhibiting the channel in their usual way.[47]
Pharmacokinetics
Amantadine is well absorbed orally. The onset of action is usually within 48 hours when used for parkinsonian syndromes, including dyskinesia. As plasma concentrations of amantadine increase, there is a greater risk for toxicity.[48][49]
Half-life elimination averages eight days in patients with end-stage renal disease. Amantadine is only minimally removed by hemodialysis.[49][50]
Amantadine is metabolized to a small extent (5-15%) by acetylation. It is mainly excreted (90%) unchanged in urine by kidney excretion.[48]
Chemistry
Amantadine is the organic compound 1-adamantylamine or 1-aminoadamantane, which consists of an adamantane backbone with an amino group substituted at one of the four methyne positions.[7] Rimantadine is a closely related adamantane derivative with similar biological properties;[51] both target the M2 proton channel of influenza A virus.[8]
History
Influenza A
Antiviral properties were first reported in 1963 at the University of Illinois Hospital in Chicago. In this amantadine trial study volunteer college students were exposed to a viral challenge. The group that received amantadine (100 milligrams 18 hours before viral challenge) had less Asia influenza infections than the placebo group.[52] Amantadine received approval for the treatment of influenza virus A[53][54][55][56] in adults in 1976.[52] It was first used in West Germany in 1966. Amantadine was approved by the U.S. Food and Drug Administration in October 1968, as a prophylactic agent against Asian (H2N2) influenza and received approval for prophylactic use for influenza A in 1976.[52][57][58]
During the 1980 influenza A epidemic, the first amantadine-resistance influenza viruses were reported. The frequency of amantadine resistance among influenza A (H3N2) viruses from 1991 and 1995 was as low as 0.8%. In 2004 the resistance frequency increased to 12.3%. A year later resistance increase significantly to 96%, 72%, and 14.5% in China, South Korea, and the United States, respectively. By 2006, 90.6% of H3N2 strains and 15.6% of H1N1 were amantadine-resistant. A majority of the amantadine-resistant H3N2 isolates (98.2%) were found to contain an S31N mutation in the M2 transmembrane domain that confers resistance to amantadine.[59] Currently, adamantane resistance is high among circulating influenza A viruses. Thus, they are no longer recommended for treatment of influenza A.[60]
Parkinson's
An incidental finding in 1969 prompted investigations about amantadine's effectiveness for treating symptoms of Parkinson's disease.[52] A woman with Parkinson's disease was prescribed amantadine to treat her influenza infection and reported her cogwheel rigidity and tremors improved. She also reported that her symptoms worsened after she finished the course of amantadine.[52] The published case report was not initially corroborated by any other instances by the medical literature or manufacturer data. A team of researchers looked at a group of ten patients with Parkinson's disease and gave them amantadine. Seven of the ten patients showed improvement, which was convincing evidence for the need of a clinical trial. The 1969 trial (lead author Robert S. Schwab, MD) included 163 patients with Parkinson's disease and 66% experienced subjective or objective reduction of symptoms with a maximum daily dose of 200 mg.[52][61] Additional studies from Schwab's team followed patients for greater lengths of time and in different combinations of neurological drugs.[62] It was found to be a safe drug that could be used over long periods of time with few side effects, as monotherapy or in combination with L-dopa or anti-cholinergic drugs.[52] By April 1973, the U.S. Food and Drug Administration approved amantadine for use in the treatment of Parkinson's disease.[63][52]
In 2017, the U.S. Food and Drug Administration approved the use of amantadine in an extended release formulation developed by Adamas Pharma for the treatment of dyskinesia, an adverse effect of levodopa in patients with Parkinson's disease.[64][65]
Veterinary use
In 2005, Chinese poultry farmers were reported to have used amantadine to protect birds against avian influenza.[66] In Western countries and according to international livestock regulations, amantadine is approved only for use in humans. Chickens in China have received an estimated 2.6 billion doses of amantadine.[66] Avian flu (H5N1) strains in China and southeast Asia are now resistant to amantadine, although strains circulating elsewhere still seem to be sensitive. If amantadine-resistant strains of the virus spread, the drugs of choice in an avian flu outbreak will probably be restricted to neuraminidase inhibitors oseltamivir and zanamivir which block the action of viral neuraminidase enzyme on the surface of influenza virus particles.[59] However, there is an increasing incidence of oseltamivir resistance in circulating influenza strains (e.g., H1N1), highlighting the need for new anti-influenza therapies.[67]
In September 2015, the U.S. Food and Drug Administration announced the recall of Dingo Chip Twists "Chicken in the Middle" dog treats because the product has the potential to be contaminated with amantadine.[68]
See also
References
- 1 2 3 4 5 6 7 8 BNF 79. London: Pharmaceutical Press. March 2020. p. 429. ISBN 978-0857113658.
- 1 2 3 4 5 6 7 8 9 "Amantadine Hydrochloride Monograph for Professionals". Drugs.com. Archived from the original on 17 November 2020. Retrieved 21 November 2020.
- 1 2 3 4 5 6 "Symmetrel (amantadine hydrochloride)" (PDF). TGA eBusiness Services. Novartis Pharmaceuticals Australia Pty Limited. 29 June 2011. Archived from the original on 12 August 2020. Retrieved 24 February 2014.
- ↑ "Trilasym 50 mg/ 5 ml Oral Solution - Summary of Product Characteristics (SmPC)". (emc). 24 September 2019. Archived from the original on 26 March 2020. Retrieved 26 March 2020.
- 1 2 Chang, C; Ramphul, K (January 2020). "Amantadine". PMID 29763128.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Amantadine Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 9 July 2021. Retrieved 21 November 2020.
- 1 2 "Amantadine". drugbank.ca. Archived from the original on 1 May 2019. Retrieved 13 July 2019.
- 1 2 3 4 5 Golan DE, Armstrong EJ, Armstrong AW (2017). Principles of pharmacology: the pathophysiologic basis of drug therapy (4th ed.). Philadelphia: Wolters Kluwer. pp. 142, 199, 205t, 224t, 608, 698–700. ISBN 9781451191004. OCLC 914593652.
- ↑ "Amantadine - an overview". ScienceDirect. Archived from the original on 27 August 2021. Retrieved 13 July 2019.
- ↑ Crosby NJ, Deane KH, Clarke CE, et al. (Cochrane Movement Disorders Group) (22 April 2003). "Amantadine for dyskinesia in Parkinson's disease". The Cochrane Database of Systematic Reviews (2): CD003467. doi:10.1002/14651858.CD003467. PMID 12804468.
- 1 2 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 242. hdl:10665/44053. ISBN 978-9241547659.
- 1 2 3 "Gocovri- amantadine capsule, coated pellets". DailyMed. 26 December 2019. Archived from the original on 1 October 2020. Retrieved 22 January 2020.
- 1 2 3 "Influenza Antiviral Medications: Summary for Clinicians". U.S. Centers for Disease Control and Prevention (CDC). 17 April 2019. Archived from the original on 13 December 2014. Retrieved 14 July 2019.
- ↑ CDC weekly influenza report – week 35 Archived 2017-10-05 at the Wayback Machine, cdc.gov
- ↑ "WHO | Summary of influenza antiviral susceptibility surveillance findings". WHO. September 2010 – March 2011. Archived from the original on 27 July 2017. Retrieved 19 July 2019.
- ↑ Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJ, et al. (Cochrane Acute Respiratory Infections Group) (November 2014). "Amantadine and rimantadine for influenza A in children and the elderly". The Cochrane Database of Systematic Reviews (11): CD002745. doi:10.1002/14651858.CD002745.pub4. PMC 7093890. PMID 25415374.
- ↑ Braley TJ, Chervin RD (August 2010). "Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment". Sleep. 33 (8): 1061–7. doi:10.1093/sleep/33.8.1061. PMC 2910465. PMID 20815187.
- ↑ Pucci E, Branãs P, D'Amico R, Giuliani G, Solari A, Taus C, et al. (Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group) (January 2007). "Amantadine for fatigue in multiple sclerosis". The Cochrane Database of Systematic Reviews (1): CD002818. doi:10.1002/14651858.CD002818.pub2. PMC 6991937. PMID 17253480.
- ↑ Payne C, Wiffen PJ, Martin S (April 2017). "WITHDRAWN: Interventions for fatigue and weight loss in adults with advanced progressive illness". The Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd. 4: CD008427. doi:10.1002/14651858.cd008427. PMC 6478103. PMID 28387447.
- ↑ Generali JA, Cada DJ (September 2014). "Amantadine: multiple sclerosis-related fatigue". Hospital Pharmacy. 49 (8): 710–2. doi:10.1310/hpj4908-710. PMC 4252198. PMID 25477595.
- ↑ Henze T, Rieckmann P, Toyka KV (2006). "Symptomatic treatment of multiple sclerosis. Multiple Sclerosis Therapy Consensus Group (MSTCG) of the German Multiple Sclerosis Society". European Neurology. 56 (2): 78–105. doi:10.1159/000095699. PMID 16966832. S2CID 5069086. Archived from the original on 2018-06-03. Retrieved 2020-11-03.
- ↑ Ma HM, Zafonte RD (February 2020). "Amantadine and memantine: a comprehensive review for acquired brain injury". Brain Injury. 34 (3): 299–315. doi:10.1080/02699052.2020.1723697. PMID 32078407. S2CID 211232548.
- ↑ Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, et al. (September 2018). "Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research". Neurology. 91 (10): 450–460. doi:10.1212/WNL.0000000000005926. PMC 6139814. PMID 30089618.
- 1 2 3 Ma HM, Zafonte RD (February 2020). "Amantadine and memantine: a comprehensive review for acquired brain injury". Brain Injury. 34 (3): 299–315. doi:10.1080/02699052.2020.1723697. PMID 32078407. S2CID 211232548.
- ↑ Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. (March 2012). "Placebo-controlled trial of amantadine for severe traumatic brain injury". The New England Journal of Medicine. 366 (9): 819–26. doi:10.1056/NEJMoa1102609. PMID 22375973.
- ↑ Hosenbocus S, Chahal R (February 2013). "Amantadine: a review of use in child and adolescent psychiatry". Journal of the Canadian Academy of Child and Adolescent Psychiatry = Journal de l'Academie Canadienne de Psychiatrie de l'Enfant et de l'Adolescent. 22 (1): 55–60. PMC 3565716. PMID 23390434. Archived from the original on 2021-04-19. Retrieved 2020-11-03.
- ↑ "Symmetrel (Amantadine Hydrochloride, USP) fact sheet" (PDF). U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 9 February 2020. Retrieved 28 July 2019.
- 1 2 3 4 "DailyMed - GOCOVRI- amantadine capsule, coated pellets". dailymed.nlm.nih.gov. Archived from the original on 1 October 2020. Retrieved 3 November 2020.
- 1 2 3 Chang C, Ramphul K (2020), "Amantadine", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763128, archived from the original on 16 December 2020, retrieved 2 November 2020
- ↑ Bahrani E, Nunneley CE, Hsu S, Kass JS (March 2016). "Cutaneous Adverse Effects of Neurologic Medications". CNS Drugs. 30 (3): 245–67. doi:10.1007/s40263-016-0318-7. PMID 26914914. S2CID 10560952. Archived from the original on 2021-08-27. Retrieved 2020-11-03.
- ↑ Vollum DI, Parkes JD, Doyle D (June 1971). "Livedo reticularis during amantadine treatment". British Medical Journal. 2 (5762): 627–8. doi:10.1136/bmj.2.5762.627. PMC 1796527. PMID 5580722. Archived from the original on 2021-04-17. Retrieved 2020-11-03.
- 1 2 3 4 "Symmetrel (Amantadine Hydrochloride, USP) fact sheet" (PDF). U.S. Food and Drug Administration. Archived (PDF) from the original on 9 February 2020. Retrieved 28 July 2019.
- ↑ Gocovri (amantadine) extended-release capsules [prescribing information]. Emeryville, CA: Adamas Pharma, LLC; August 2017
- ↑ Reglan (metoclopramide) [prescribing information]. Baudette, MN: ANI Pharmaceuticals Inc; August 2017
- ↑ Tarsy, D; Parkes, J D; Marsden, C D (1975-04-01). "Metoclopramide and pimozide in Parkinson's disease and levodopa-induced dyskinesias". Journal of Neurology, Neurosurgery & Psychiatry. 38 (4): 331–335. doi:10.1136/jnnp.38.4.331. ISSN 0022-3050. PMC 491929. PMID 1095689. Archived from the original on 2021-08-27. Retrieved 2020-11-11.
- 1 2 "Australian Product Information – Symmetrel (Amantadine Hydrochloride) Capsules". Australian Department of Health Therapeutic Goods Administration. Archived from the original on 10 July 2017. Retrieved 13 July 2019.
- ↑ "Symmetrel (Amantadine) Prescribing Information" (PDF). Endo Pharmaceuticals. May 2003. Archived from the original (PDF) on 17 November 2014. Retrieved 2 August 2007.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Chang C, Ramphul K (2020). Amantadine. StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 29763128. Archived from the original on 16 December 2020. Retrieved 2 November 2020.
- ↑ "Amantadine – MeSH". NCBI. Archived from the original on 2018-08-21. Retrieved 2018-08-04.
- ↑ Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. p. 3962. ISBN 978-3-85200-181-4.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Kornhuber J, Bormann J, Hübers M, Rusche K, Riederer P (April 1991). "Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: a human postmortem brain study". European Journal of Pharmacology. 206 (4): 297–300. doi:10.1016/0922-4106(91)90113-v. PMID 1717296.
- ↑ Blanpied TA, Clarke RJ, Johnson JW (March 2005). "Amantadine inhibits NMDA receptors by accelerating channel closure during channel block". The Journal of Neuroscience. 25 (13): 3312–22. doi:10.1523/JNEUROSCI.4262-04.2005. PMC 6724906. PMID 15800186.
- ↑ Strömberg U, Svensson TH (November 1971). "Further studies on the mode of action of amantadine". Acta Pharmacologica et Toxicologica. 30 (3): 161–71. doi:10.1111/j.1600-0773.1971.tb00646.x. PMID 5171936.
- 1 2 Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E (April 2004). "Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine". The European Journal of Neuroscience. 19 (8): 2212–20. doi:10.1111/j.0953-816X.2004.03297.x. PMID 15090047. S2CID 19479968.
- ↑ Thomaston JL, Alfonso-Prieto M, Woldeyes RA, Fraser JS, Klein ML, Fiorin G, DeGrado WF (November 2015). "High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction". Proceedings of the National Academy of Sciences of the United States of America. 112 (46): 14260–5. Bibcode:2015PNAS..11214260T. doi:10.1073/pnas.1518493112. PMC 4655559. PMID 26578770.
- ↑ PubChem. "Amantadine". pubchem.ncbi.nlm.nih.gov. Archived from the original on 1 May 2019. Retrieved 29 July 2019.
- ↑ Hussain, Mazhar; Galvin, Henry D.; Haw, Tatt Y.; Nutsford, Ashley N.; Husain, Matloob (2017-04-20). "Drug resistance in influenza A virus: the epidemiology and management". Infection and Drug Resistance. 10: 121–134. doi:10.2147/idr.s105473. PMC 5404498. PMID 28458567. Archived from the original on 2020-11-12. Retrieved 2020-11-09.
- 1 2 "Amantadine Hydrochloride Monograph for Professionals". Drugs.com. Archived from the original on 2020-11-17. Retrieved 2020-11-16.
- 1 2 "Amantadine - FDA prescribing information, side effects and uses". Drugs.com. Archived from the original on 2020-11-03. Retrieved 2020-11-16.
- ↑ Deleu, Dirk; Northway, Margaret G.; Hanssens, Yolande (2002). "Clinical Pharmacokinetic and Pharmacodynamic Properties of Drugs Used in the Treatment of Parkinson??s Disease". Clinical Pharmacokinetics. 41 (4): 261–309. doi:10.2165/00003088-200241040-00003. ISSN 0312-5963. Archived from the original on 2021-08-27. Retrieved 2020-11-17.
- ↑ "Rimantadine hydrochloride (CHEBI:8865)". ebi.ac.uk. Archived from the original on 15 February 2020. Retrieved 13 July 2019.
- 1 2 3 4 5 6 7 8 Hubsher G, Haider M, Okun MS (April 2012). "Amantadine: the journey from fighting flu to treating Parkinson disease". Neurology. 78 (14): 1096–9. doi:10.1212/WNL.0b013e31824e8f0d. PMID 22474298. S2CID 21515610.
- ↑ Hounshell DA, Kenly Smith J (1988). Science and Corporate Strategy: Du Pont R&D, 1902–1980. Cambridge University Press. p. 469. ISBN 978-0521327671. Archived from the original on 2016-04-28. Retrieved 2016-01-05.
- ↑ "Sales of flu drug by du Pont unit a 'disappointment'". The New York Times. Wilmington, Delaware. 5 October 1982. Archived from the original on 27 August 2021. Retrieved 19 May 2008.
- ↑ Maugh TH (November 1979). "Panel urges wide use of antiviral drug". Science. 206 (4422): 1058–60. Bibcode:1979Sci...206.1058M. doi:10.1126/science.386515. PMID 386515.
- ↑ Maugh TH (April 1976). "Amantadine: an alternative for prevention of influenza". Science. 192 (4235): 130–1. doi:10.1126/science.192.4235.130. PMID 17792438.
- ↑ "Gocovri- amantadine capsule, coated pellets". DailyMed. 26 December 2019. Archived from the original on 1 October 2020. Retrieved 22 January 2020.
- ↑ "International Review of Neurobiology", Recent advances in the use of Drosophila in neurobiology and neurodegeneration, International Review of Neurobiology, vol. 99, Elsevier, 2011, pp. i–iii, doi:10.1016/b978-0-12-387003-2.00010-0, ISBN 978-0-12-387003-2, archived from the original on 2021-08-27, retrieved 2020-11-11
- 1 2 Kumar, Binod; Asha, Kumari; Khanna, Madhu; Ronsard, Larance; Meseko, Clement Adebajo; Sanicas, Melvin (2018-04-01). "The emerging influenza virus threat: status and new prospects for its therapy and control". Archives of Virology. 163 (4): 831–844. doi:10.1007/s00705-018-3708-y. ISSN 1432-8798. PMC 7087104. PMID 29322273. Archived from the original on 2021-08-27. Retrieved 2020-11-11.
- ↑ "Antiviral Drug Resistance among Influenza Viruses | CDC". www.cdc.gov. 2019-04-17. Archived from the original on 2014-02-13. Retrieved 2020-11-11.
- ↑ Schwab, Robert S. (19 May 1969). "Amantadine in the Treatment of Parkinson's Disease". JAMA: The Journal of the American Medical Association. 208 (7): 1168. doi:10.1001/jama.1969.03160070046011. ISSN 0098-7484.
- ↑ Schwab RS, Poskanzer DC, England AC, Young RR (November 1972). "Amantadine in Parkinson's disease. Review of more than two years' experience". JAMA. 222 (7): 792–5. doi:10.1001/jama.222.7.792. PMID 4677928.
- ↑ "Symmetrel (Amantadine Hydrochloride, USP) fact sheet" (PDF). U.S. Food and Drug Administration. Archived (PDF) from the original on 9 February 2020. Retrieved 28 July 2019.
- ↑ Bastings E. "NDA 208944 Approval Letter" (PDF). Archived (PDF) from the original on 2019-06-17. Retrieved 2017-08-28.
- ↑ "Drug Approval Package: Gocovri (amantadine extended-release)". U.S. Food and Drug Administration. 29 June 2018. Archived from the original on 29 October 2020. Retrieved 22 January 2020.
- 1 2 Sipress A (18 June 2005). "Bird Flu Drug Rendered Useless". The Washington Post. Archived from the original on 14 August 2014. Retrieved 2 August 2007.
- ↑ Aoki FY, Boivin G, Roberts N (2007). "Influenza virus susceptibility and resistance to oseltamivir". Antiviral Therapy. 12 (4 Pt B): 603–16. PMID 17944268.
- ↑ "Enforcement Report – Week of September 23, 2015". fda.gov. U.S. Food and Drug Administration. Archived from the original on October 2, 2015. Retrieved September 30, 2015.
External links
| Identifiers: |
|---|
- "Amantadine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2017-01-24. Retrieved 2020-01-09.
- "Amantadine hydrochloride". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2017-02-16. Retrieved 2020-10-20.