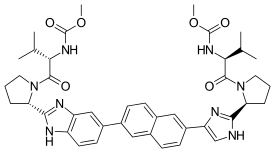
Ravidasvir
 | |
| Names | |
|---|---|
| Other names | PPI-668 |
IUPAC name
| |
| Clinical data | |
| Drug class | NS5A inhibitor[1] |
| Main uses | Hepatitis C[2] |
| Side effects | Generally minor, low blood sugar[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth[2] |
| Chemical and physical data | |
| Formula | C42H50N8O6 |
| Molar mass | 762.912 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ravidasvir is a medication used to treat hepatitis C.[2] It is typically used together with sofosbuvir with a 97% cure rate.[4] It can be used in people infected with both hepatitis C and HIV/AIDS.[4] It is taken by mouth.[2]
Side effects are generally minor.[3] In people with diabetes, low blood sugar may occur.[3] In those who also have hepatitis B reactivation may occur.[3] It is a NS5A inhibitor.[1]
Ravidasvir was approved for medical use in Malaysia and Egypt in 2021.[4] It is on the World Health Organization's List of Essential Medicines.[5] A 12 week course of treatment is expected to cost about 300 to 500 USD.[1]
History
Preliminary clinical trial results were announced in Nov 2015.[6] In April 2017, press reports stated that a combination treatment involving ravidasvir and sofosbuvir had achieved a 97% clearup rate against hepatitis C in a clinical trial conducted in Malaysia and Thailand, and 100% in another conducted in Egypt.[7] It has been granted conditional registration by the National Pharmaceutical Regulatory Agency (NPRA) of Malaysia.[8][9]
References
- 1 2 3 "First hepatitis C treatment developed through South-South cooperation registered in Malaysia | DNDi". dndi.org. 14 June 2021. Archived from the original on 7 April 2023. Retrieved 10 September 2023.
- 1 2 3 4 "eEML - Electronic Essential Medicines List". list.essentialmeds.org. Archived from the original on 10 September 2023. Retrieved 10 September 2023.
- 1 2 3 4 "24th WHO Expert Committee on Selection and Use of Essential Medicines Expert review" (PDF). WHO. WHO. Archived (PDF) from the original on 10 September 2023. Retrieved 10 September 2023.
- 1 2 3 "Ravidasvir + sofosbuvir | DNDi". dndi.org. 31 December 2015. Archived from the original on 21 May 2023. Retrieved 10 September 2023.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Good Results for Sovaldi and Ravidasvir Treating Hepatitis C Genotype 4". Hepatitis Magazine. November 24, 2015. Archived from the original on June 4, 2023. Retrieved August 16, 2023.
- ↑ Kollewe J (13 April 2018). "Non-profit's $300 hepatitis C cure as effective as $84,000 alternative". The Guardian. Archived from the original on 25 May 2023. Retrieved 16 August 2023.
- ↑ "List of new products approved by the National Pharmaceutical Regulatory Agency (NPRA) of Malaysia" (PDF). Archived (PDF) from the original on 2022-08-08. Retrieved 2023-08-16.
- ↑ "First hepatitis C treatment developed through South-South cooperation registered in Malaysia | DNDi". dndi.org. 2021-06-14. Archived from the original on 2021-08-10. Retrieved 2021-08-10.
External links
| Identifiers: |
|---|