Zanamivir
 | |
| Names | |
|---|---|
| Pronunciation | /zəˈnæmɪvɪər/ |
| Trade names | Relenza, Dectova, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Neuraminidase inhibitor[1] |
| Main uses | Influenza[1] |
| Side effects | Headache, dizziness, bronchitis, cough[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Inhalation, IV |
| Typical dose | 10 mg twice daily[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 2% (by mouth) |
| Protein binding | <10% |
| Metabolism | Negligible |
| Elimination half-life | 2.5–5.1 hours |
| Excretion | Kidney |
| Chemical and physical data | |
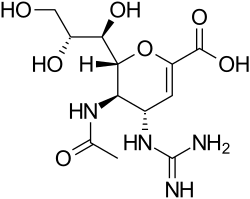
| Formula | C12H20N4O7 |
| Molar mass | 332.313 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Zanamivir, sold under the brand name Relenza among others, is a medication used to treat and prevent influenza due to influenza A or B viruses.[1] It is used by breathing in the powder or by injection into a vein.[1][3]
Common side effects include headache, dizziness, bronchitis, and cough.[1] Use is not recommended in those with asthma or COPD.[1] While it does not appear to cause harm in pregnancy or breastfeeding, it has not been well studied in this population.[4] It is a neuraminidase inhibitor and works by stopping viral replication.[1]
Zanamivir was approved for medical use in the United States in 1999.[1] One course of treatment in the United States costs about 68 USD as of 2021.[5] This amount in the United Kingdom costs the NHS about £16.[2] The injectable form is available in Europe.[3]
Medical uses
Zanamivir is used for the treatment of infections caused by influenza A and influenza B viruses, but in otherwise-healthy individuals, benefits overall appear to be small. It decreases the risk of one's getting symptomatic, but not asymptomatic influenza. The combination of diagnostic uncertainty, the risk for virus strain resistance, possible side effects and financial cost outweigh the small benefits of zanamivir for the prophylaxis and treatment of healthy individuals.[6]
Since then, genes expressing resistance to zanamivir were found in Chinese people infected with avian influenza A H7N9 during treatment with zanamivir.[7]
Treatment
In otherwise-healthy individuals, benefits overall appear to be small.[6] Zanamivir shortens the duration of symptoms of influenza-like illness (unconfirmed influenza or 'the flu') by less than a day. In children with asthma there was no clear effect on the time to first alleviation of symptoms.[8] Whether it affects the risk of one's need to be hospitalized or the risk of death is not clear.[6] There is no proof that zanamivir reduced hospitalizations or pneumonia and other complications of influenza, such as bronchitis, middle ear infection, and sinusitis.[8][9] Zanamivir did not reduce the risk of self reported investigator mediated pneumonia or radiologically confirmed pneumonia in adults. The effect on pneumonia in children was also not significant.[10]
Prevention
Low to moderate evidence indicates it decreases the risk of one's getting influenza by 1% to 12% in those exposed.[6] Prophylaxis trials showed that zanamivir reduced the risk of symptomatic influenza in individuals and households, but there was no evidence of an effect on asymptomatic influenza or on other, influenza-like illnesses. Also there was no evidence of reduction of risk of person-to-person spread of the influenza virus.[8] The evidence for a benefit in preventing influenza is weak in children, with concerns of publication bias in the literature.[11]
Resistance
As of 2009, no influenza had shown any signs of resistance in the US.[12] A meta-analysis from 2011 found that zanamivir resistance had been rarely reported.[13] Antiviral resistance can emerge during or after treatment with antivirals in certain people (e.g., immunosuppressed).[14] In 2013 genes expressing resistance to zanamivir (and oseltamivir) were found in Chinese patients infected with avian influenza A H7N9.[7]
Dosage
As treatment it is used as 10 mg twice per day for five days.[2]
Side effects
Dosing is limited to the inhalation route. This restricts its usage, as treating asthmatics could induce bronchospasms.[15] In 2006 the Food and Drug Administration (FDA) found that breathing problems (bronchospasm), including deaths, were reported in some patients after the initial approval of Relenza. Most of these patients had asthma or chronic obstructive pulmonary disease. Relenza therefore was not recommended for treatment or prophylaxis of seasonal influenza in individuals with asthma or chronic obstructive pulmonary disease.[16] In 2009 the zanamivir package insert contains precautionary information regarding risk of bronchospasm in patients with respiratory disease.[17] GlaxoSmithKline (GSK) and FDA notified healthcare professionals of a report of the death of a patient with influenza having received zanamivir inhalation powder, which was solubilized and administered by mechanical ventilation.[18]
In adults there was no increased risk of reported adverse events in trials. There was little evidence of the possible harms associated with the treatment of children with zanamivir.[8] Zanamivir has not been known to cause toxic effects and has low systemic exposure to the human body.[19]
Mechanism of action
Zanamivir works by binding to the active site of the neuraminidase protein, rendering the influenza virus unable to escape its host cell and infect others.[20] It is also an inhibitor of influenza virus replication in vitro and in vivo. In clinical trials, zanamivir was found to reduce the time-to-symptom resolution by 1.5 days if therapy was started within 48 hours of the onset of symptoms.
The bioavailability of zanamivir is 2%. After inhalation, zanamivir is concentrated in the lungs and oropharynx, where up to 15% of the dose is absorbed and excreted in urine.[21]
History
Zanamivir was first made in 1989 by scientists led by Peter Colman[22][23] and Joseph Varghese[24] at the Australian CSIRO, in collaboration with the Victorian College of Pharmacy, and the Monash University. Zanamivir was the first of the neuraminidase inhibitors. The discovery was initially funded by the Australian biotechnology company Biota and was part of Biota's ongoing program to develop antiviral agents through rational drug design. Its strategy relied on the availability of the structure of influenza neuraminidase by X-ray crystallography. It was also known, as far back as 1974, that 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA), a sialic acid analogue, is an inhibitor of neuraminidase.[25]
Computational chemistry techniques were used to probe the active site of the enzyme, in an attempt to design derivatives of DANA that would bind tightly to the amino acid residues of the catalytic site, so would be potent and specific inhibitors of the enzyme. The GRID software by Molecular Discovery was used to determine energetically favourable interactions between various functional groups and residues in the catalytic site canyon. This investigation showed a negatively charged zone occurs in the neuraminidase active site that aligns with the C4 hydroxyl group of DANA. This hydroxyl is, therefore, replaced with a positively charged amino group; the 4-amino DANA was shown to be 100 times better as an inhibitor than DANA, owing to the formation of a salt bridge with a conserved glutamic acid (119) in the active site. Glu 119 was also noticed to be at the bottom of a conserved pocket in the active site that is just big enough to accommodate the larger, but more basic guanidine functional group.[26] Zanamivir, a transition-state analogue inhibitor of neuraminidase, was the result.[27]
As Biota was a small company, it did not have the resources to bring zanamivir to market by itself. In 1990, zanamivir patent rights were licensed to Glaxo, now GlaxoSmithKline (GSK). The license agreement entitled Biota to receive a 7% royalty on Glaxo's sales of zanamivir.
In 1999, the product was approved for marketing in the US and Europe for treatment of influenza A and B. The FDA advisory committee had recommended by a vote 13 to 4 that it should not be approved, because it lacked efficacy and was no more effective than placebo when the patients were on other drugs such as paracetamol. But the FDA leadership overruled the committee and criticised its reviewer, biostatistician Michael Elashoff. The review of oseltamivir, which was also in approval process at that time, was taken away from him, and reassigned to someone else.[28] In 2006 zanamivir was approved in the US and Europe for prevention of influenza A and B.[29]
Although zanamivir was the first neuraminidase inhibitor to the market, it had only a few months lead over the second entrant, oseltamivir (Tamiflu), with an oral capsule formulation.
When first marketed in the US in 1999/2000, zanamivir captured only 25% of the influenza antiviral market, despite a huge promotional campaign. By the end of that season, Tamiflu was outselling zanamivir 3:1. During that season, zanamivir experienced worldwide safety warnings involving the risk of bronchospasm and death. Glaxo then reduced the marketing of zanamivir, and Tamiflu's dominance increased. More than US$20 million worth of zanamivir sold by Glaxo in the first US season was returned to the company in the next two seasons because its sales to patients were far less than expected.
Biota commenced legal proceedings in 2004 alleging Glaxo's reduced marketing of zanamivir to be a breach of contract. Biota claimed about A$700 million from Glaxo. After Biota spent four years trying to progress its case, and incurring A$50 million in legal costs, the company abandoned the claim in July 2008, recovering only A$20 million, including legal costs following settlement at mediation. Biota had refused an earlier tactical offer from Glaxo of A$75 million plus legal costs.
In August 2006, Germany announced it would buy 1.7 million doses of zanamivir, as part of its preparation strategy against bird flu. "Germany's purchase shows that countries are starting to take a balanced view of influenza preparedness," said Simon Tucker, head of research at Melbourne-based Biota, where zanamivir was originally developed.[20]
In April 2009, many cases of swine flu (H1N1-type virus) were reported in US and Mexico. Zanamivir is one of only two drugs prescribed to treat it. A study published in June 2009 emphasized the urgent need for augmentation of oseltamivir stockpiles, with additional antiviral drugs including zanamivir, based on an evaluation of the performance of these drugs in the scenario that the 2009 H1N1 swine flu neuraminidase (NA) were to acquire the Tamiflu-resistance (His274Tyr) mutation, which is currently widespread in 99.6% of all tested seasonal H1N1 strains.[30]
In January 2011, GSK announced it would commence phase III trials for intravenous zanamivir in a study that will span 20 countries in the Northern and Southern Hemispheres.[31]
References
- 1 2 3 4 5 6 7 8 9 "Zanamivir Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 4 August 2021.
- 1 2 3 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 700. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 "Dectova". Archived from the original on 2 January 2021. Retrieved 4 August 2021.
- ↑ "Zanamivir (Relenza) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 4 August 2021.
- ↑ "Zanamivir Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 12 June 2016. Retrieved 4 August 2021.
- 1 2 3 4 Michiels, B.; Van Puyenbroeck, K.; Verhoeven, V.; Vermeire, E.; Coenen, S. (2013). "The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews". PLOS One. 8 (4): e60348. Bibcode:2013PLoSO...860348M. doi:10.1371/journal.pone.0060348. PMC 3614893. PMID 23565231.
- 1 2 Hu, Y.; Lu, S.; Song, Z.; Wang, W.; Hao, P.; Li, J.; Zhang, X.; Yen, H. L.; Shi, B.; Li, T.; Guan, W.; Xu, L.; Liu, Y.; Wang, S.; Zhang, X.; Tian, D.; Zhu, Z.; He, J.; Huang, K.; Chen, H.; Zheng, L.; Li, X.; Ping, J.; Kang, B.; Xi, X.; Zha, L.; Li, Y.; Zhang, Z.; Peiris, M.; Yuan, Z. (2013). "Association between adverse clinical outcome in human disease caused by novel influenza a H7N9 virus and sustained viral shedding and emergence of antiviral resistance". The Lancet. 381 (9885): 2273–2279. doi:10.1016/S0140-6736(13)61125-3. PMID 23726392.
- 1 2 3 4 Jefferson, T; Jones, MA; Doshi, P; Del Mar, CB; Hama, R; Thompson, MJ; Spencer, EA; Onakpoya, I; Mahtani, KR; Nunan, D; Howick, J; Heneghan, CJ (10 April 2014). "Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children" (PDF). The Cochrane Database of Systematic Reviews. 4 (4): CD008965. doi:10.1002/14651858.CD008965.pub4. PMC 6464969. PMID 24718923. Archived (PDF) from the original on 14 May 2021. Retrieved 22 March 2021.
- ↑ Heneghan, CJ; Onakpoya, I; Thompson, M; Spencer, EA; Jones, M; Jefferson, T (Apr 9, 2014). "Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments". BMJ (Clinical Research Ed.). 348: g2547. doi:10.1136/bmj.g2547. PMC 3981976. PMID 24811412.
- ↑ Heneghan, C. J.; Onakpoya, I.; Thompson, M.; Spencer, E. A.; Jones, M.; Jefferson, T. (9 April 2014). "Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments". BMJ. 348 (apr09 2): g2547. doi:10.1136/bmj.g2547. PMC 3981976. PMID 24811412.
- ↑ Wang, K; Shun-Shin, M; Gill, P; Perera, R; Harnden, A (Apr 18, 2012). "Neuraminidase inhibitors for preventing and treating influenza in children (published trials only)". Cochrane Database of Systematic Reviews. 4 (4): CD002744. doi:10.1002/14651858.CD002744.pub4. PMC 6599832. PMID 22513907.
- ↑ "2008-2009 Influenza Season Week 32 ending August 15, 2009". Flu Activity & Surveillance. Centers for Disease Control and Prevention (CDC). August 21, 2009. Archived from the original on July 9, 2017. Retrieved March 22, 2021.
- ↑ Thorlund, Kristian; Awad, Tahany; Boivin, Guy; Thabane, Lehana (2011). "Systematic review of influenza resistance to the neuraminidase inhibitors". BMC Infectious Diseases. 11 (1): 134. doi:10.1186/1471-2334-11-134. PMC 3123567. PMID 21592407.
- ↑ "Influenza Antiviral Medications: Summary for Clinicians". CDC. 2018-05-11. Archived from the original on 2014-12-13. Retrieved 21 April 2014.
- ↑ Hayden FG (December 2001). "Perspectives on antiviral use during pandemic influenza". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 356 (1416): 1877–84. doi:10.1098/rstb.2001.1007. PMC 1088564. PMID 11779387.
- ↑ "FDA Approves a Second Drug for the Prevention of Influenza A and B in Adults and Children". FDA press release. Archived from the original on 2010-03-06. Retrieved 2021-03-22.
- ↑ "Safe and Appropriate Use of Influenza Drugs". Public Health Advisories (Drugs). U.S. Food and Drug Administration (FDA). April 30, 2009. Archived from the original on 2009-11-04. Retrieved 2009-11-11.
- ↑ "Archive copy". Archived from the original on 2017-01-18. Retrieved 2021-03-22.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Freund, B; Gravenstein, S; Elliott, M; Miller, I (Oct 1999). "Zanamivir: a review of clinical safety". Drug Safety. 21 (4): 267–81. doi:10.2165/00002018-199921040-00003. PMID 10514019.
- 1 2 Cyranoski D (September 2005). "Threat of pandemic brings flu drug back to life". Nature Medicine. 11 (9): 909. doi:10.1038/nm0905-909. PMID 16145557.
- ↑ Moscona A (September 2005). "Neuraminidase inhibitors for influenza". The New England Journal of Medicine. 353 (13): 1363–73. doi:10.1056/NEJMra050740. PMID 16192481. Archived from the original on 2021-08-29. Retrieved 2021-03-22.
- ↑ Varghese, J. N.; Laver, W. G.; Colman, P. M. (1983). "Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 a resolution". Nature. 303 (5912): 35–40. doi:10.1038/303035a0. PMID 6843658.
- ↑ "Archived copy". Archived from the original on October 4, 2013. Retrieved October 2, 2013.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archived copy". Archived from the original on October 5, 2013. Retrieved October 2, 2013.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Meindl P, Bodo G, Palese P, Schulman J, Tuppy H (April 1974). "Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid". Virology. 58 (2): 457–63. doi:10.1016/0042-6822(74)90080-4. PMID 4362431.
- ↑ Laver, Graeme (1 March 2007). "Flu drugs - pathway to discovery". Education in Chemistry. Vol. 44, no. 2. Royal Society of Chemistry. pp. 48–52. ISSN 0013-1350. Archived from the original on 19 June 2018. Retrieved 19 June 2018.
- ↑ von Itzstein, M; Wu, WY; Kok, GB; Pegg, MS; Dyason, JC; Jin, B; Van Phan, T; Smythe, ML; White, HF; Oliver, SW (3 June 1993). "Rational design of potent sialidase-based inhibitors of influenza virus replication". Nature. 363 (6428): 418–23. Bibcode:1993Natur.363..418V. doi:10.1038/363418a0. PMID 8502295.
- ↑ Cohen, D.; Carter, P. (3 June 2010). "WHO and the pandemic flu "conspiracies"". BMJ. 340 (jun03 4): c2912. doi:10.1136/bmj.c2912. PMID 20525679.
- ↑ "FDA Approves a Second Drug for the Prevention of Influenza A and B in Adults and Children FDA press release March 29, 2006". FDA. Archived from the original on March 6, 2010. Retrieved March 22, 2021.
- ↑ Soundararajan V, Tharakaraman K, Raman R, Raguram S, Sasisekharan V, Sasisekharan R (June 2009). "Extrapolating from sequence--the 2009 H1N1 'swine' influenza virus". Nature Biotechnology. 27 (6): 510–3. doi:10.1038/nbt0609-510. PMID 19513050.
- ↑ Hirschler, Ben (2011-01-19). "GSK tests intravenous flu drug vs Roche's Tamiflu". Reuters. Archived from the original on 2021-08-27. Retrieved 2021-03-22.
External links
| Identifiers: |
|---|
- The development of Relenza for treatment of influenza
- Drug Information: Zanamivir Inhalation Archived 2016-07-05 at the Wayback Machine MedlinePlus drug
- Relenza: Consumer Questions and Answers Archived 2017-07-22 at the Wayback Machine US Food and Drug Administration (FDA)