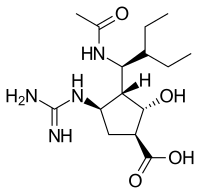
Peramivir
 | |
| Names | |
|---|---|
| Trade names | Rapivab |
IUPAC name
| |
| Clinical data | |
| Drug class | Neuraminidase inhibitor[1] |
| Main uses | Influenza[1] |
| Side effects | Low neutrophils, trouble sleeping, nausea[2][1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Typical dose | 10 mg/kg up to 600 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100% (IV) |
| Elimination half-life | 7.7 to 20.8 hours (in patients with normal renal function) |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H28N4O4 |
| Molar mass | 328.413 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Peramivir, sold under the brand name Rapivab, is an antiviral used to treat influenza.[1] It should be given within two days of the start of symptoms.[1] Evidence does not support its use for prevention.[1] It is given by gradual injection in to a vein.[2]
Common side effects include low neutrophils, trouble sleeping, and nausea.[2][1] Other side effects may include abnormal behavior.[1] Serious side effects may include anaphylaxis and Stevens-Johnson syndrome.[2] It does not appear to be harmful in pregnancy, but such use has not been well studied.[3] It is a neuraminidase inhibitor that is active against both influenza A and B viruses.[1]
Peramivir was approved for medical use in the United States in 2014.[1] While it was approved in Europe in 2018, this approval was subsequently withdrawn.[4] In the United States it costs about 1,000 USD per dose as of 2021.[5]
Medical use
Dosage
It is given at a dose of 600 mg once per day for 1 to 5 days in adults and at a dose of 10 mg/kg in children.[1]
History
An intramuscular (IM) peramivir phase II study for seasonal influenza in 2008–2009 found no effect for the primary endpoint of improvement in the median time to alleviation of symptoms in subjects with confirmed, acute, uncomplicated influenza infection versus placebo.
On October 23, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization for peramivir, allowing the use of the drug in intravenous form for hospitalized patients only in cases where the other available methods of treatment are ineffective or unavailable;[6][7][8] for instance, if oseltamivir resistance develops and a person is unable to take zanamivir via the inhaled route. The U.S. government (department of Health and Human Services) gave BioCryst Pharmaceuticals more than $77 million to finish the Phase III clinical development of peramivir. In 2009 the department of Health and Human Services had already given about $180 million to the program.[9] Biocryst also donated 1200 courses of treatment to the US department of Health and Human Services.[10] The Emergency Use Authorization expired on June 23, 2010. In 2011 a phase III trial found the median durations of influenza symptoms were the same with 1 intravenous injection of peramivir against 5 days of oral oseltamivir for people with seasonal influenza virus infection.[11]
In 2012 BioCryst reported that it should halt enrollment on its study for intravenous peramivir in potentially life-threatened people after an interim analysis led trial monitors to conclude that it would be futile to continue and the trial should be terminated. The difference between peramivir and control group (oral oseltamivir) for the primary endpoint, clinical or virologic, was small.[12] In 2013 the Biomedical Advanced Research and Development Authority (BARDA/HHS) released new funding under the current $234.8 million contract to enable completion of a New Drug Application filing for intravenous (IV) peramivir.[13]
According to a research report published in June 2011, a new variant of swine flu had emerged in Asia with a genetic adaptation (a S247N neuraminidase mutation) giving some resistance to oseltamivir and zanamivir, but no significant reduction in sensitivity to peramivir.[14][15] But a H274Y virus mutation showed resistance to oseltamivir and peramivir, but not to zanamivir, and only in N1 neuraminidases.[16] Ultimately 3.2% (19/599) of A(H1N1)pdm09 viruses collected between 2009 and 2012 had highly reduced peramivir inhibition due to the H275Y NA mutation.[17]
BioCryst Pharmaceuticals submitted a new drug application (NDA) to the U.S. Food and Drug Administration (FDA) for intravenous peramivir in December 2013.[18] Peramivir (Rapivab) was approved for intravenous administration in December 2014.[19][20]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Peramivir Monograph for Professionals". Drugs.com. Archived from the original on 7 October 2020. Retrieved 27 October 2021.
- 1 2 3 4 "Alpivab (peramivir)" (PDF). Archived (PDF) from the original on 6 March 2020. Retrieved 27 October 2021.
- ↑ "Peramivir (Rapivab) Use During Pregnancy". Drugs.com. Archived from the original on 24 November 2020. Retrieved 27 October 2021.
- ↑ "Alpivab". Archived from the original on 26 June 2021. Retrieved 27 October 2021.
- ↑ "Rapivab Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 April 2021. Retrieved 27 October 2021.
- ↑ "Emergency Use Authorization Granted For BioCryst's Peramivir". Reuters. 2009-10-24. Archived from the original on 2009-10-27.
- ↑ Thorlund K, Awad T, Boivin G, Thabane L (May 2011). "Systematic review of influenza resistance to the neuraminidase inhibitors". BMC Infectious Diseases. 11 (1): 134. doi:10.1186/1471-2334-11-134. PMC 3123567. PMID 21592407.
- ↑ "Peramivir authorized for Emergency use". LifeHugger. 2009-12-04. Archived from the original on 2011-07-13. Retrieved 2009-12-04.
- ↑ "Feds hand BioCryst $77M for anti-viral trial". Fierce biotech. September 21, 2009. Archived from the original on 2016-03-04. Retrieved 2021-08-09.
- ↑ "FDA Authorizes Emergency Use of Intravenous Antiviral Peramivir for 2009 H1N1 Influenza for Certain Patients, Settings". Reuters. 2009-10-24. Archived from the original on 2009-10-27.
- ↑ Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, et al. (November 2011). "Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection". Antimicrobial Agents and Chemotherapy. 55 (11): 5267–76. doi:10.1128/AAC.00360-11. PMC 3195028. PMID 21825298.
- ↑ "BioCryst scraps $235M late-stage flu drug program backed by feds". Fierce Biotech. November 8, 2012. Archived from the original on 2016-03-03. Retrieved 2021-08-09.
- ↑ "BioCryst to File Peramivir NDA Supported by BARDA/HHS Funding". Fierce Biotech. July 11, 2013. Archived from the original on March 4, 2016. Retrieved August 9, 2021.
- ↑ Hurt, A.C. (9 June 2011). "Increased detection in Australia and Singapore of a novel influenza A(H1N1)2009 variant with reduced oseltamivir and zanamivir sensitivity due to a S247N neuraminidase mutation". Eurosurveillance. Archived from the original on 23 April 2014. Retrieved 9 August 2021.
- ↑ Hirschler, Ben (2011-06-10). "Swine flu starting to show resistance to drugs". Reuters. Archived from the original on 2016-03-06. Retrieved 2021-08-09.
- ↑ McKimm-Breschkin JL (January 2013). "Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance". Influenza and Other Respiratory Viruses. 7 Suppl 1: 25–36. doi:10.1111/irv.12047. PMC 4942987. PMID 23279894.
- ↑ Leang SK, Kwok S, Sullivan SG, Maurer-Stroh S, Kelso A, Barr IG, Hurt AC (March 2014). "Peramivir and laninamivir susceptibility of circulating influenza A and B viruses". Influenza and Other Respiratory Viruses. 8 (2): 135–9. doi:10.1111/irv.12187. PMC 4186459. PMID 24734292.
- ↑ "BioCryst Files Peramivir NDA for the Treatment of Influenza" (Press release). BioCryst Pharmaceuticals. 2013-12-20. Archived from the original on 2021-02-25. Retrieved 2021-08-09.
- ↑ "Drug Approval Package: Rapivab (peramivir) Injection NDA #206426". U.S. Food and Drug Administration (FDA). 16 January 2015. Archived from the original on 11 February 2020. Retrieved 11 February 2020.
- ↑ "Rapivab: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 11 February 2020. Retrieved 11 February 2020.
External links
| Identifiers: |
|---|
- "Peramivir". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-09-17. Retrieved 2021-08-09.
- "Peramivir: Requirements for Administration under EUA". Lifehugger. Archived from the original on 2011-07-13.