Uprifosbuvir
 | |
| Clinical data | |
|---|---|
| Trade names | Uprifosbuvir |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
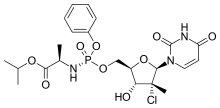
| Formula | C22H29ClN3O9P |
| Molar mass | 545.9 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Uprifosbuvir (MK-3682) is an antiviral drug developed for the treatment of Hepatitis C. It is a nucleotide analogue which acts as an NS5B RNA polymerase inhibitor. It is currently in Phase III human clinical trials.[1][2][3]
References
- ↑ Soriano V, Fernandez-Montero JV, de Mendoza C, Benitez-Gutierrez L, Peña JM, Arias A, Barreiro P (August 2017). "Treatment of hepatitis C with new fixed dose combinations". Expert Opinion on Pharmacotherapy. 18 (12): 1235–1242. doi:10.1080/14656566.2017.1346609. PMID 28644739. S2CID 205819421.
- ↑ Borgia G, Maraolo AE, Nappa S, Gentile I, Buonomo AR (March 2018). "NS5B polymerase inhibitors in phase II clinical trials for HCV infection". Expert Opinion on Investigational Drugs. 27 (3): 243–250. doi:10.1080/13543784.2018.1420780. PMID 29271672. S2CID 3672885.
- ↑ Lawitz E, Gane E, Feld JJ, Buti M, Foster GR, Rabinovitz M, et al. (September 2019). "Efficacy and safety of a two-drug direct-acting antiviral agent regimen ruzasvir 180 mg and uprifosbuvir 450 mg for 12 weeks in adults with chronic hepatitis C virus genotype 1, 2, 3, 4, 5 or 6". Journal of Viral Hepatitis. 26 (9): 1127–1138. doi:10.1111/jvh.13132. PMID 31108015. S2CID 160014275.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.